Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.28 no.4 Madrid jul./ago. 2013
https://dx.doi.org/10.3305/nh.2013.28.4.6494
ORIGINAL/Otros
Predicting survival of endoscopic gastrostomy candidates using the underlying disease, serum cholesterol, albumin and transferrin levels
Predicción de supervivencia en candidatos a gastrostomía endoscópica haciendo uso de la enfermedad de base del paciente y de los niveles plasmáticos de colesterol, albúmina y transferrina
Jorge Fonseca, Carla Adriana Santos and José Brito
Hospital García de Orta. Lisboa. Portugal
ABSTRACT
Background: Endoscopic gastrostomy (PEG) is the gold standard for long-term enteral feeding. An adequate PEG candidate must have life expectancy longer than a few weeks. Patients surviving less than three weeks should have a nasogastric tube, and gastrostomy should be avoid. There are few studies looking to prognostic factors and fewer attempts of creating a predictor model for PEG patient's survival.
Aim: The aim of this study was creating a predictive survival model for PEG candidates, using underlying disease, cholesterol, albumin and transferrin.
Methods: Data was obtained from records of adult patients that underwent PEG between 1999 and 2011. Patients surviving < 3 weeks were considered short survivors; surviving > 3 weeks were considered adequate survivors. A full logistic regression model was used to classify future cases into one of the two groups of survival.
Results: An equation for the probability of future cases was generated, in order to obtain a P value. In the future, patients with a P > 0,88 will have a 64,7% probability of adequate surviving; patients with a P < 0,88 will have a 70.3% probability of short surviving.
Conclusions: When clinical evaluation alone does not display a clear prognosis, this equation should be included in the evaluation of gastrostomy candidates, avoiding useless gastrostomy.
Key words: Gastrostomy. Prognosis. Underlying disease. Albumin. Transferrin. Cholesterol.
RESUMEN
Introducción: La Gastrostomia Endoscópica Percutánea (PEG) es una técnica de elección para la nutrición enteral de largo plazo, pero en pacientes con una perspectiva de vida menor que tres semanas debe evitarse la gastrostomia e utilizarse la sonda nasogástrica. Existen pocos estudios que tengan en cuenta factores de pronóstico y pocos intentos de crear un modelo predictivo de supervivencia de estos pacientes.
Objetivo: Crear un modelo predictivo de la sobrevida de los candidatos a PEG, utilizando la enfermedad de base, los niveles de colesterol, albúmina y transferrina.
Métodos: Los datos han sido recogidos de los registros clínicos de adultos sometidos a PEG entre 1999 y 2011. Los que han sobrevivido menos de 3 semanas se han considerado como sin supervivencia adecuada (short survivors); los que han sobrevivido más de 3 semanas se consideraran como teniendo sobrevivencia adecuada (adequate survivors). Se utilizó un modelo estadístico de regresión para clasificar pacientes futuros en cada uno de los grupos definidos anteriormente.
Resultados: De este análisis estadístico resultó una ecuación que permite obtener un valor de P, para determinar la probabilidad de futuros casos. En el futuro se puede predecir que pacientes con un valor de P > 0,88 tendrán 64,7% de probabilidades de supervivencia adecuada; los pacientes con un valor de P < 0,88 tendrán 70,3% de probabilidades de supervivencia no adecuada.
Conclusiones: Cuando la evaluación clínica por si sola no es suficiente para predecir un pronóstico fiable, esta ecuación debe ser incluida en la evaluación de los pacientes candidatos a gastrostomia, para evitar gastrostomías inútiles.
Palabras clave: Gastrostomia. Pronóstico. Enfermedad. Albúmina. Transferrina. Colesterol.
Introduction
Percutaneous endoscopic gastrostomy (PEG) is the gold standard for long-term enteral feeding. Candidates for PEG placement are mainly dysphagic patients. For most enteral nutrition teams,1 according to our experience,2 most cases of dysphagia are caused by neurological disease or by head and neck cancer. These patients frequently have speech and motor impairments, asymmetric muscle atrophy and malnutrition, all induced by dysphagia and/or systemic underlying diseases. The standard nutritional evaluation tools may not be adequate to evaluate most of these patients and enteral feeding teams must frequently rely on anthropometric and laboratory data in order to monitor the nutritional status evolution of these patients.
On the search for easily available laboratory markers that may reflect the nutritional status and also help estimate prognosis of PEG patients with a wide spectrum of underlying diseases, we focused on serum albumin, serum transferrin and total serum cholesterol levels. Serum albumin was one of the first3 and most used laboratory measurements for assessing nutritional status and prognosis in a large number of clinical settings as diverse as hemodialysis,4 pancreatitis5 and cancer.6 There is a large consensus about the effectiveness of albumin in the evaluation of nutritional status in elder patients,7,8 and it shows a good correlation with the Mini Nutritional Assessment,8 a nutritional assessment tool especially designed for the elderly. Interestingly, albumin has also proved to be useful for nutritional evaluation and prognosis in an internal medicine department with a very wide range of diseases.9 In its turn, serum transferrin has long been used in nutritional and prognostic assessment,10,11 and in a wide variety of clinical settings as well, including hemodialysis,12 cancer,13 pancreatitis14 and intensive care patients.15 Low serum transferrin is associated with poor nutrition and poor outcome in elder patients.16 Finally, low serum cholesterol is a well known marker of under nutrition and poor prognosis17 and has been associated with shorter survival in several epidemiological studies since the 90's18 (Harris et al, 1992). It has been associated with poor outcome in a broad range of clinical conditions, namely hemodialysis19 and heart failure20 in critical ill21 and elderly patients.7,22 Serum total cholesterol, albumin and transferrin are useful markers for all these patients and in many other clinical settings. These laboratory markers are under the bias of nutritional, inflammatory and other disease-related factors, which makes it often difficult to separate all those influences. But it is precisely this aptitude of accurately marking a poor outcome, regardless of the involved mechanisms, that makes total cholesterol, albumin and transferrin so attractive to use in gastrostomy patients, a group with a broad spectrum of underlying disorders.
An adequate PEG candidate should fulfill the three following conditions: except for proximal lesion, the digestive tube should be undamaged, endoscopy and endoscopic transillumination must be feasible and life expectancy should be longer than a few weeks. The traditional requirement was a life expectancy of 4-6 weeks and this standard is still used by some groups.1 Nevertheless, a shorter life expectancy, of three or even two weeks23, is the criterion accepted by most teams taking care of enteral feeding patients.
As PEG placing procedure is associated with mild morbidity and a very small number (< 1%) of procedure-associated deaths, patients with very poor prognosis are better managed with a nasogastric tube. In our enteral feeding team, we seldom advocate PEG placement in patients in whom we anticipate a life expectancy shorter than three weeks. Although it is critical for gastrostomy procedure decision, estimation of patient prognosis relies mainly on clinical judgment. Some objective data that may support this decision would be most useful. Nevertheless, there are only a small number of studies regarding prognostic factors for PEG patient's survival. We found only few attempts of creating a predictor model for PEG patient's survival. A study of 502 hospitalized patients from nursing homes who underwent PEG identified low serum albumin (< 3 g/dl), chronic obstructive pulmonary disease and diabetes mellitus as risk factors.24 In a study of 198 patients, most of them (75.3%) with cerebrovascular disorders, low serum albumin and history of pneumonia before the procedure were associated with poor prognosis.25 In an observational prospective study with 160 patients, Moreno Pérez et al., indentified some independent prognostic factors for patients in hospital setting.26 Chicharro et al. (2009) identified some patient's factors that may help the selection for gastrostomy, but only in elder patients.27 In a recent study, higher age, lower BMI, and diabetes mellitus were related to worst outcomes.28 The Sheffield Gastrostomy Score (SGS) devised to try to improve outcomes following this procedure, using age and serum albumin to try predicting 30-day mortality risk in a cohort of patients that included stroke, oropharyngeal malignancy and neurodegenerative diseases. It was created in Sheffield and validated in four hospitals of the same region,29 but never reached a widespread acceptance. Certainly, the wide range of underlying diseases of dysphagic PEG patients is an obstacle for designing a global prognosis model.
The aim of the present study was to evaluate the possibility of predicting survival of PEG candidates, and therefore avoiding erroneous gastrostomy, using simple data as the underlying disease and three widespread serum markers, total serum cholesterol, serum albumin and serum transferrin.
Patients and methods
The study population included adult patients (> 18 years) that underwent endoscopic gastrostomy in our hospital between November 1st, 1999 and April 30th, 2011. Patients were referred by their clinicians and evaluated together by the enteral nutrition team in order to establish the clinical indication for gastrostomy. Informed consent for endoscopic gastrostomy and for clinical and laboratory initial evaluation was obtained from every patient or from their relative/legal responsible. Serum total cholesterol (mg/dl), albumin (g/dl) and serum transferrin (mg/dl) were part of standard initial evaluation. In the day of the endoscopic procedure, before the PEG tube placement, a blood sample was taken for laboratory evaluation.
Clinical, laboratory and demographic data were obtained from medical records. Patients with incomplete records were excluded. Collected data included age, gender, underlying disease, survival after gastrostomy, and albumin, transferrin and total cholesterol levels on the day of the gastrostomy. Underlying diseases were divided into three groups: Group 1, neurological diseases; Group 2, head and neck cancers and Group 3, other diseases.
Survival was evaluated in days, from the day of the endoscopic gastrostomy until the day the patient died or until May 31st, 2011, if the patient was alive. Patients surviving less than 3 weeks (< 21 days) were considered short survivors (SS). Gastrostomy should have not been performed in most of them had this short survival time been anticipated and they could have been fed through a nasogastric tube. Patients surviving 3 weeks or more (> 21 days) were considered adequate survivors (AS) and, retrospectively, PEG feeding was considered the correct option.
Statistical analysis of the data was performed using the software SPSS® 19.0. A full logistic regression model was used in order to classify future cases into one of the two survival groups, SS or AS. All statistical tests were performed with a 5% level of significance.
Results
Descriptive statistics
From our clinical files we selected 298 patients with complete medical records, which included 203 men and 95 women. Neurological disease was the cause of dysphagia in 165 patients, 126 had head and neck cancer and 7 suffered from other diseases.
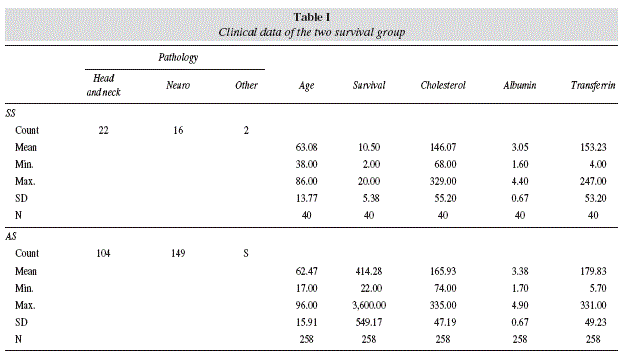
Age ranged from 18 to 86 years. Median age was 63 (men: 61, women: 89). Initial total cholesterol ranged from 68 to 335 mg/dl (mean: 163.4). Initial transferrin ranged from 4 to 331 mg/dl (mean: 176.1). Initial albumin ranged from 1.5 to 4.8 g/dl (mean: 3.3). Survival range from 2 to 3600 days (median: 82). Table I displays clinical and laboratorial features of both SS and AS groups.
Logistic regression analysis of the survival data
A full logistic regression model was used to classify future cases into one of the two survival groups defined above as a function of serum cholesterol, transferrin and albumin, and type of underlying disease.
Prior analysis of the data has shown that, in the variable set considered for this study, only albumin, cholesterol and transferrin have the potential to discriminate between groups, if strict adherence to the 5% level of statistical significance is considered, with higher serum levels being observed amongst adequate survivors. Instead, that potential was strongly suggested for the type of pathology but not for gender or age. Since pathology is an important variable in predicting survival, it was decided to include this variable in the model. Since this is a discrete, nominal scaled variable, it is inappropriate to include it the model as if it were interval scaled.
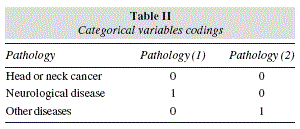
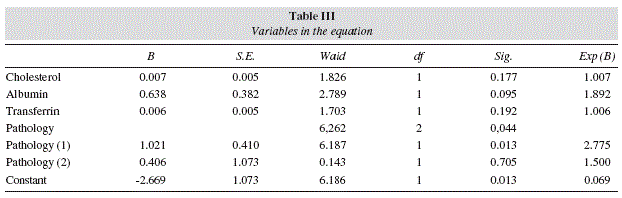
Consequently, two design variables were generated to represent the type of pathology, namely pathology (1) and pathology (2), using the indicator contrast method with the reference category defined by the head or neck cancer pathology. Table 2 shows the codes used to define these two design variables. In the first run of the model to the full data set, the type of underlying disease was the only suggested predictor of survival (p = 0.070), with an overall poor fit, as revealed by the Hosmer-Lemeshow test (p = 0.023) and the Nagelkerke R2 (0.107). Moreover, 3 individual patterns were identified by model residuals in excess of 0.95, which turned out to be of considerable influence. In effect, when those 3 observations were removed from calculations, the fitness improved somehow, as shown by the clearly non-significant result of the Hosmer-Lemeshow test (p = 0.292) and the Nagelkerke R2 (0.157). More importantly, the type of underlying disease emerges now as a clearer and significant predictor of survival (p = 0.044), while the benefits of higher serum albumin towards longer survival are also suggested (p < 0.1). Moreover, of the two design variables used to represent the type of pathology, only pathology(1) reaches statistical significance, and thus predicting increased survival in cases of neurological disease when compared to head or neck cancers. Table 3 presents the structure of the model obtained in these conditions.
With the data in the table above, the following equation for the posterior probability of future cases of adequate survival, P, can be used:
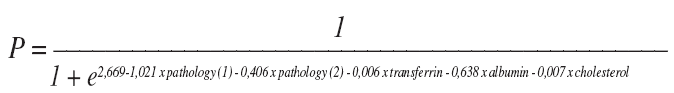
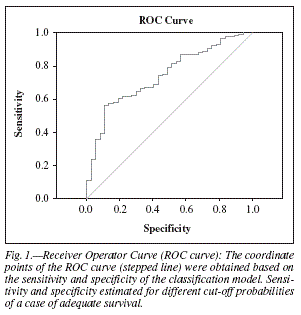
In order to evaluate the performance of the classification scheme defined by the above equation, a ROC curve analysis was implemented (fig. 1). The asymptotic significance is below 0.05, which means that using the logistic regression model to predict future cases is better than just guessing. Moreover, the estimated area under the curve is 0.742, which represents the probability of a model's result for a randomly chosen positive case exceeding the result for a randomly chosen negative case.
In order to select a cutoff point for the classification of future cases using the logistic model which warrants acceptable sensitivity and specificity, the coordinates of the ROC curve were analyzed. The best choice would be to use as a cutoff point the percentage of adequate survivors suggested by the sample, which is estimated as 87.5%. Thus, if a person is classified as an adequate survivor given the probability predicted by the model, P, is greater than or equal to 0.880, then the overall percentage of correctly classified future cases would be 65.4%, with 70.3% of short survivors and 64.7% of adequate survivors being correctly classified. That means that, in the future, any patient with a P > 0,88 will have a 64,7% probability of surviving 3 weeks or more and any patient with a P < 0,88 will have a 70.3% probability of surviving less them 3 weeks.
Discussion
Most Parenteral and Enteral Nutrition (PEN) societies advocate gastrostomy if enteral feeding is expected to be needed for more than a few weeks. The USA PEN society, ASPEN, suggests 4 weeks30 while ESPEN advocates 2-3 weeks.31 Recent guidelines from the French health high authority for nutritional support in the elderly advice gastrostomy if swallowing disorders persist beyond 2 weeks.23 Nevertheless, these and other guidelines and recommendations never propose any method of prognostic evaluation, relying on clinical judgment alone. Every enteral feeding team keeps a record of a number of patients dying a few days after the gastrostomy. In most of these patients, the procedure was useless and should have not been performed. Actually, prognosis estimation is a critical issue of gastrostomy candidate's evaluation. Mortality analysis is an important issue32 because the gastrostomy would be denied in most patients surviving less than a few weeks, if this outcome could be anticipated.
The present study used three routine laboratory tests, serum cholesterol, albumin and transferrin. When considered separately, each of these serum markers showed a significant difference between the two survival groups, with higher serum levels being observed in longer survival, while such contrast was only suggested for the type of pathology. This different behavior shown by the four variables may as well reflect the different statistical power of the data analysis techniques used, t tests for the biochemical markers, and exact Fisher's test, for type of pathology. However, when the four variables were simultaneously considered in the logistic regression model, type of pathology was the only significant predictor of adequate survival, presenting itself as a surrogate measure of those serum parameters. Nevertheless, laboratory data are still important for survival estimation in our model. Other variables were evaluated, including total lymphocyte count or clinical parameters but there was no improvement of the model. In the future, other centers and other enteral feeding teams may use this model, increasing the number of patients enrolled and confirming the validity of the model in various clinical settings.
Knowing the underlying disease (pathology group) with these laboratory data and a simple equation,
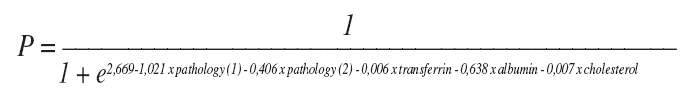
we can predict if a patient will survive more than three weeks and has a satisfactory life expectancy to be considered for gastrostomy. Conversely, if the patient has a life expectancy shorter than three weeks, gastrostomy should seldom be considered, only on the basis of very special, individual features. PEG patients and patients dying shortly after gastrostomy are a heterogeneous group, in respect to age, underlying diseases, comorbidities and nutritional status. Nevertheless, our model is robust and there seems to be no differences between the three groups of different underlying diseases. As a matter of fact, the present model is useful independently of the heterogeneity of the gastrostomy patients. Globally, this model is effective in preventing most of the gastrostomy procedures in short survivors, but it proves itself to be wrong in a minority of patients and should not be used as a golden rule. Some patients with P > 0,88, with expectable adequate survival may pass away within 3 weeks. In these patients PEG would be useless. Conversely, some patients with P < 0,88, with expectable short survival, may be alive for more than 3 weeks. These ones can be fed through a nasogastric tube for a few weeks more and PEG will just be delayed. Furthermore, even in situations that clearly have a very poor outcome, an endoscopic gastrostomy may be considered in order to provide the most comfortable palliation. Obviously, this equation is no substitute for clinical evaluation. The decision process must be based on clinical features and ethical considerations and must be shared between the enteral feeding team, the patient and the family. But, despite being an imperfect model, when patient prognosis is uncertain, the present model may become part of the evaluation of PEG candidates, as a useful tool to support clinical decisions.
Conclusions
The authors present a useful tool for predicting the outcome of gastrostomy candidates. Knowing the underlying disease of gastrostomy candidates, and evaluating total serum cholesterol, serum albumin and serum transferrin and using an equation, enteral feeding teams should be able to identify most of the gastrostomy candidates that would survive less than three weeks after the procedure, and avoid useless gastrostomy procedures. When clinical evaluation alone does not display a clear prognosis, this equation should be included in the evaluation of gastrostomy candidates.
References
1. Ortega PR, Continente AC, Puertas MJM, Alcántara CG, López PB, Hernández RL. Gastrostomia Endoscópica Percutánea: 7 años de experiencia en nutrición enteral a largo plazo. Seguimiento clínico. Nutr Hosp 2011; 26 (2): 399-401. [ Links ]
2. Fonseca J, Santos C. Clinical data evolution and survival of 200 consecutive PEG patients (abstract). Clin Nutr 2009; 4 (Suppl.): 122-3. [ Links ]
3. Jones RE, Schendel HE.Nutritional status of selected Negro infants in Greenville County, South Carolina. Am J Clin Nutr 1966; 18: 407-12. [ Links ]
4. Thijssen S, Usvyat L, Kotanko P. Prediction of Mortality in the First Two Years of Hemodialysis: Results from a Validation Study.Blood Purif 2012; 33: 165-70. [ Links ]
5. Pavlidis TE, Pavlidis ET, Sakantamis AK. Advances in prognostic factors in acute pancreatitis: a mini-review. Hepatobiliary Pancreat Dis Int 2010; 9: 482-6. [ Links ]
6. Elahi MM, McMillan DC, McArdle CS, Angerson WJ, Sattar N. Score based on hypoalbuminemia and elevated C-reactive protein predicts survival in patients with advanced gastrointestinal cancer. Nutr Cancer 2004; 48: 171-3. [ Links ]
7. Schalk BW, Visser M, Deeg DJ, Bouter LM. Lower levels of serum albumin and total cholesterol and future decline in functional performance in older persons: the Longitudinal Aging Study Amsterdam. Age Ageing 2004; 33 (3): 266-72. [ Links ]
8. Kagansky N, Berner Y, Koren-Morag N, Perelman L, Knobler H, Levy S. Poor nutritional habits are predictors of poor outcome in very old hospitalized patients. Am J Clin Nutr 2005; 82: 784-91. [ Links ]
9. Numeroso F, Barilli AL, Delsignore R. Prevalence and significance of hypoalbuminemia in an internal medicine department. Eur J Intern Med 2008; 19: 587-91. [ Links ]
10. McFarlane H, Reddy S, Cooke A, Longe O, Onabamiro MO, Houba JE. Immunoglobulins, transferrin, caeruloplasmin and heterophile antibodies in kwashiorkor. Trop Geogr Med 1970; 22: 61-4. [ Links ]
11. Burritt MF, Anderson CF. Laboratory assessment of nutritional status. Hum Pathol 1984; 15: 30-3. [ Links ]
12. Jahromi SR, Hosseini S, Razeghi E, Meysamie A, Sadrzadeh H. Malnutrition predicting factors in hemodialysis patients. Saudi J Kidney Dis Transpl 2010; 21: 846-51. [ Links ]
13. Guerra LT, Rosa AR, Romani RF, Gurski RR, Schirmer CC, Kruel CD. Serum transferrin and serum prealbumin as markers of response to nutritional support in patients with esophageal cancer. Nutr Hosp 2009; 24: 241-2. [ Links ]
14. Doley RP, Yadav TD, Wig JD, Kochhar R, Singh G, Bharathy KG, Kudari A, Gupta R, Gupta V, Poornachandra KS, Dutta U, Vaishnavi C. Enteral nutrition in severe acute pancreatitis. JOP 2009; 10: 157-62. [ Links ]
15. Kim H, Choi-Kwon S. Changes in nutritional status in ICU patients receiving enteral tube feeding: a prospective descriptive study. Intensive Crit Care Nurs 2011; 27: 194-201. [ Links ]
16. Donini LM, De Felice MR, Savina C, Coletti C, Paolini M, Laviano A, Scavone L, Neri B, Cannella C. Predicting the outcome of long-term care by clinical and functional indices: the role of nutritional status. J Nutr Health Aging 2011; 15: 586-92. [ Links ]
17. González Madroño A, Mancha A, Rodríguez FJ, de Ulibarri JI, Culebras J. The use of biochemical and immunological parameters in nutritional screening and assessment. Nutr Hosp 2011; 26: 594-601. [ Links ]
18. Harris T, Feldman JJ, Kleinman JC, Ettinger WH Jr, Makuc DM, Schatzkin AG.The low cholesterol-mortality association in a national cohort. J Clin Epidemiol 1992; 45: 595-601. [ Links ]
19. Iseki K, Yamazato M, Tozawa M, Takishita S. Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int 2002; 61: 1887-93. [ Links ]
20. Horwich TB, Hamilton MA, Maclellan WR, Fonarow GC. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail 2002; 8: 216-24. [ Links ]
21. Vyroubal P, Chiarla C, Giovannini I, Hyspler R, Ticha A, Hrnciarikova D, Zadak Z. Hypocholesterolemia in clinically serious conditions-review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2008; 152: 181-9. [ Links ]
22. Hrnciarikova D, Hyspler R, Vyroubal P, Klemera P, Hronek M, Zadak Z. Serum lipids and neopterin in urine as new biomarkers of malnutrition and inflammation in the elderly. Nutrition 2009; 25: 303-8. [ Links ]
23. Raynaud-Simon A, Revel-Delhom C, Hébuterne X; French Nutrition and Health Program, French Health High Authority. Clinical practice guidelines from the French Health High Authority: nutritional support strategy in protein-energy malnutrition in the elderly. Clin Nutr 2011; 30: 312-9. [ Links ]
24. Lang A, Bardan E, Chowers Y, Sakhnini E, Fidder HH, Bar-Meir S, Avidan B.Risk factors for mortality in patients undergoing percutaneous endoscopic gastrostomy. Endoscopy 2004; 36 (6): 522-6. [ Links ]
25. Tokunaga T, Kubo T, Ryan S, Tomizawa M, Yoshida S, Takagi K, Furui K, Gotoh T. Long-term outcome after placement of a percutaneous endoscopic gastrostomy tube. Geriatr Gerontol Int 2008; 8: 19-23. [ Links ]
26. Pérez OM, Avilés AM, Martínez A, Boix E, Aznar S, Martín MD, et al. Factores pronósticos de morbi-mortalidad en nutrición enteral hospitalaria: estudio prospective. Nutr Hosp 2005; 3: 210-6. [ Links ]
27. Chicharro L, Puiggroós I, Pérez-Portabella C, Planas M. Complicaciones inmediatas de la gastrostomía Percutánea de alimentación: 10 años de experiencia. Nutr Hosp 2009; 24 (1): 73-6. [ Links ]
28. Zopf Y, Maiss J, Konturek P, Rabe C, Hahn EG, Schwab D. Predictive factors of mortality after PEG insertion: guidance for clinical practice. J Parenter Enteral Nutr 2011; 35: 50-5. [ Links ]
29. Kurien M, Robson HE, Leeds JS, Grant J, McAlindon ME, Dear KL, Hoeroldt BS, Kapur K, James G, Sanders DS. External validation of a prognostic scoring system for percutaneous endoscopic gastrostomy (PEG). (abstract) Gut 2011; 60: A17. [ Links ]
30. Bankhead R, Boullata J, Brantley S, Corkins M, Guenter P, Krenitsky J, Lyman B, Metheny NA, Mueller C, Robbins S, Wessel J; ASPEN. Board of Directors. Enteral nutrition practice recommendations. J Parenter Enteral Nutr 2009; 33: 122-67. [ Links ]
31. Löser C, Aschl G, Hébuterne X, Mathus-Vliegen EM, Muscaritoli M, Niv Y, Rollins H, Singer P, Skelly RH. ESPEN guidelines on artificial enteral nutrition-percutaneous endoscopic gastrostomy (PEG). Clin Nutr 2005; 24: 848-61. [ Links ]
32. Longcroft-Wheaton G, Marden P, Colleypriest B, Gavin D, Taylor G, Farrant M. Understanding why patients die after gastrostomy tube insertion: a retrospective analysis of mortality. J Parenter Enteral Nutr 2009; 33: 375-9. [ Links ]
![]() Correspondence:
Correspondence:
Jorge Fonseca
Hospital García de Orta
Av. Prof. Torrado da Silva, Pragal
2800 Almada. Lisboa. Portugal
E-mail: jorgedafonseca@hotmail.com
Recibido: 10-II-2013
Aceptado: 13-V-2013