INTRODUCTION
Type-2 diabetes mellitus (T2DM) has been defined as a metabolic disease that develops due to a progressive loss of adequate β-cell insulin secretion, frequently in the setting of insulin resistance.
It accounts for 90-95 % of all diabetes cases and includes individuals with insulin deficiency and/or peripheral insulin resistance (1). The prevalence of T2DM is growing in many developing and most developed countries. The International Diabetes Federation (IDF) reported that there were 463 million people with diabetes worldwide in 2019 and that this number will increase to 700 million by 2045 (2). The cause of T2DM is poorly understood, but insulin resistance causing β-cell loss or dysfunction appears to be the main reason for T2DM development (1).
Fetuin-A, also called Alpha 2-Heremans Schmid Glycoprotein (AHSG), is an endogenous glycoprotein predominantly secreted by the liver (3). First discovered in 1944, it has been reported to have a protective effect from ectopic calcium deposition and vascular calcification. Since then, it has been shown to be a multifunctional protein with effects on multiple cellular pathways and associated with the skeletal system, cardiovascular system, metabolism, and nervous system (4). In recent years, emerging evidence suggests that fetuin-A plays a role in insulin resistance, especially in individuals with T2DM, and is an independent predictor of type-2 diabetes (3-5).
One of the effector mechanisms of fetuin-A on insulin resistance and diabetes development is that fetuin-A is a natural endogenous inhibitor of the insulin-stimulated insulin receptor tyrosine kinase (6). In epidemiological studies, it has been shown that there is a relationship between serum fetuin-A level and the risk of developing insulin resistance and diabetes (6,7). Another mechanism that may explain the relationship between high fetuin-A levels and insulin resistance is that fetuin-A causes inflammation by inducing inflammatory cytokines, which in turn causes insulin sensitivity (8). Previous studies have shown that fetuin-A induces the expression of pro-inflammatory cytokines (8,9).
In recent years, diet has been identified as an important modulator of chronic inflammation (10). Besides, it has been suggested that diet may also increase T2DM risk via inflammation (11). In this regard, many nutrients with anti-inflammatory effects have been associated with a lower risk of T2DM whereas nutrients with pro-inflammatory effects have been associated with a higher risk of T2DM in previous studies (12,13).
There is an increasing number of studies investigating the effect of the inflammatory potential of diet on diseases, and the dietary inflammatory index (DII) has been widely used in these studies (14,15). DII is a literature-derived, validated index that was designed to measure the inflammatory potential of diet. Regarding the index, a higher DII score represents a pro-inflammatory diet, whereas a lower score represents an anti-inflammatory diet (16). Besides, only a few studies have investigated the relationship between the inflammatory potential of diet as measured by DII and T2DM (14,17). To the best of our knowledge, no studies have focused on the mediator role of fetuin-A in this relationship. In addition, limited studies are evaluating the effect of fetuin-A on T2DM.
The present study aimed to investigate the effect of an inflammatory diet on the risk of developing T2DM via fetuin-A, an acute phase reactant, and to determine the effect of fetuin-A on T2DM risk.
MATERIALS AND METHODS
STUDY POPULATION
This case-control study included a total of 80 patients aged between 30 and 50 years, with 40 obese women (BMI, 30-35) with T2DM as the case group and 40 obese women (BMI, 30-35) as the control group. The study sample size was calculated using the G*Power, version 3.1.9.2, software package, taking into account the results of previous studies; the error rate was 0.05 and power was 80 %. Individuals who attended the Family Medicine Outpatient Clinic of Health Sciences University Dışkapı Yıldırım Beyazıt Training and Research Hospital, referred to the Nutrition and Dietetics Department, and matching inclusion criteria were included in the study. The individuals excluded from the study were as follows; type-1 diabetes patients, type-2 diabetes patients receiving insulin treatment, those in the menopause period, pregnant or breastfeeding women, those with acute or chronic inflammatory diseases, liver or kidney failure, severe psychiatric disorder, cancer patients, those who received steroid/antibiotic treatment, anti-inflammatory drugs, medication for obesity for the previous six months, and those who regularly used drugs (except oral antidiabetics).
The study protocol was reviewed by the Clinical Research Ethics Committee of University of Health Sciences, Dışkapı Yıldırım Beyazıt Training and Research Hospital and approved by the report of the decision number 70/04 on 26.08.2019. All participants provided a written informed consent.
ANTHROPOMETRIC MEASUREMENTS
Anthropometric measurements, including height, weight, waist and, hip circumference, were measured with as few and as thin clothes as possible and without shoes. These measurements were done by the researcher in the morning. Body height (m) was measured using a stadiometer attached to the digital weight scale (Seca 769). To measure weight (kg), a digital weight scale with an accuracy of 0.1 kg (Seca 769) was used. Body mass index (BMI) was calculated as weight over height squared (kg/m2). Individuals between 30 and 35 kg/m2 were included in the study, considering the World Health Organization (WHO) criteria (18). Waist circumference (cm) was measured at the midpoint between the inferior margin of the last rib and the iliac crest with an inelastic tape in the horizontal plane. Hip circumference (cm) was also measured at the same position, using the same tape at the widest point of the hip. The waist-hip ratio was calculated by dividing the waist circumference of the participants by their hip circumference.
ASSESSMENT OF DIETARY INTAKE AND DIETARY INFLAMMATORY INDEX
The dietary intake of individuals was assessed using a quantitative food frequency questionnaire (QFFQ). The consumed amount of each food was multiplied by the specific coefficient of frequency of consumption, and the average daily amounts of the foods were obtained. The Nutrient Database Programme (BeBiS, Ebispro for Windows, Germany; Turkish Version/BeBiS 8.2) was used to determine average daily energy and nutrient intake for each individual. To measure the dietary inflammatory potential of the diet, the dietary inflammatory index (DII), which is a valid and reliable tool, was used. The calculation steps of the DII have been previously described in detail in its methods paper (16). Briefly, the DII is based on a literature review and analysis of articles published up to 2010 linking dietary components with inflammatory markers, including TNF-α, CRP, IL-1β, IL-4, IL-6, and IL-10. Each dietary parameter associated with inflammation in the articles was scored according to its effects on these six inflammatory markers. Each was assigned a ‘food parameter-specific inflammatory effect score'. A global data set including these inflammation-related food parameters' standard global daily intake (mean values and standard deviations of 45 food parameters) and food parameter-specific inflammatory effect score was created. The DII is calculated using this global composite database. When calculating the DII, firstly the inflammatory effect score is calculated separately for each food parameter consumed by the individuals in the dataset, then the sum of these obtained scores represents the inflammatory index score of an individual's diet. In this study, DII was calculated for each individual as follows: Z-scores were obtained by subtracting the standard global mean from the reported amount and dividing that value by the standard deviation. To reduce the effects of right-skewing (a common occurrence with dietary data), Z-scores for each food parameter were converted to percentiles. The percentile score is converted to the center percentile score [(percentile score x 2) - 1]. The central percentile score for each intake parameter was multiplied by the "food parameter-specific inflammatory effect score". The overall DII was calculated by summing the inflammatory effect scores calculated for each nutrient parameter consumed by the individual. In this study, 44 of the original 45 DII food parameters were available from the FFQ and were used for DII calculation. These included mean daily intakes of energy, carbohydrates, fiber, protein, total fat, saturated fat, monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), omega-3 fatty acids, omega-6 fatty acids, cholesterol, alcohol, vitamin A, vitamin D, vitamin E, vitamin B1 vitamin B2, niacin, vitamin B6, vitamin B12, folic acid, vitamin C, beta-carotene, magnesium, iron, zinc, selenium, flavon-3-ols, flavones, flavonols, flavanones, anthocyanidins, isoflavones, caffeine, tea, onion, garlic and pepper, eugenol, ginger, thyme, rosemary, turmeric, and saffron. Higher DII scores (positive or close to positive) represent a pro-inflammatory diet quality and lower DII scores (negative or close to negative) represent an anti-inflammatory diet quality.
SERUM COLLECTION AND LABORATORY MEASUREMENTS
Fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), fasting insulin, white blood cell (WBC), lymphocyte (LYM), neutrophile (NEU), triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and hs-CRP levels were analyzed using standard techniques from blood samples taken after an overnight fast (at least 8 hours). The homeostatic model assessment of insulin resistance (HOMA-IR) value, the indicator of insulin resistance, was calculated with the following formula: fasting blood glucose (mg/dL) x fasting insulin (µU/mL) / 405. Blood samples (10 mL, 2 gel tubes), which were taken to analyze serum fetuin-A, IL-6, and TNF-α levels, were centrifuged and preserved at −80 ºC with the serum separated until the analysis. Fetuin-A (Human Fetuin-A ELISA BioVendor Laboratories, Modrice, Czech Republic) (Catalog No.: RD191037100), IL-6 (DIAsource ImmunoAssays S.A., Belgium) (Catalog No.: KAP1261), and TNF-α (DIAsource ImmunoAssays S.A., Belgium) (Catalog No.: KAP1751) were measured using enzyme-linked immunosorbent assay (ELISA) test kits according to the manufacturer's protocol.
STATISTICAL ANALYSIS
Chi-square tests were used for categorical variables, and Student's t-test and Mann-Whitney U-test were used for continuous variables to evaluate differences between different groups. Definitive statistics were presented as mean () ± standard deviation (SD) or median, minimum-maximum (min-max) or frequency, percentage (%), where appropriate. Spearman's correlation analysis was performed to assess the strength of the relationship between continuous variables. Binary logistic regression was used to evaluate the relationship between some predictors and T2DM risk. The simple mediation analysis as described by Preacher and Hayes (19) was used to investigate whether the DII was associated with glucose metabolism markers or whether the association was mediated by fetuin-A and other inflammatory markers (Fig. 1). Total, direct, and indirect effects of DII on glucose metabolism markers were evaluated by using independent, dependent, and mediator variables. Path "c" shows the ‘total effect' of DII on glucose metabolism markers without adjusting mediator variables (Fig. 1A); the product of regression coefficients α and β (αβ) shows the ‘indirect effect' (mediated effect) of DII on glucose metabolism markers via the mediator variables (Fig. 1B); path "c'" shows the ‘direct effect' of DII on glucose metabolism markers after adjusting the effect of the mediator variables (Fig. 1B). When the total and indirect effects are significant, and the direct effect is non-significant (NS), a ‘full or complete mediation' occurred; when the total and indirect effects are significant, and the direct effect remains significant, a ‘partial or incomplete mediation' occurred. When both the total and indirect effects are NS, the result was designated ‘inconsistent mediation'. The mediation effect proportion was calculated using the following equation: [αβ / αβ + c']. The statistical analyses were performed using the IBM SPSS (Statistical Package for Social Sciences, SPSS Company, IL, USA) program, version 23. Statistical significance was defined as p < 0.05.
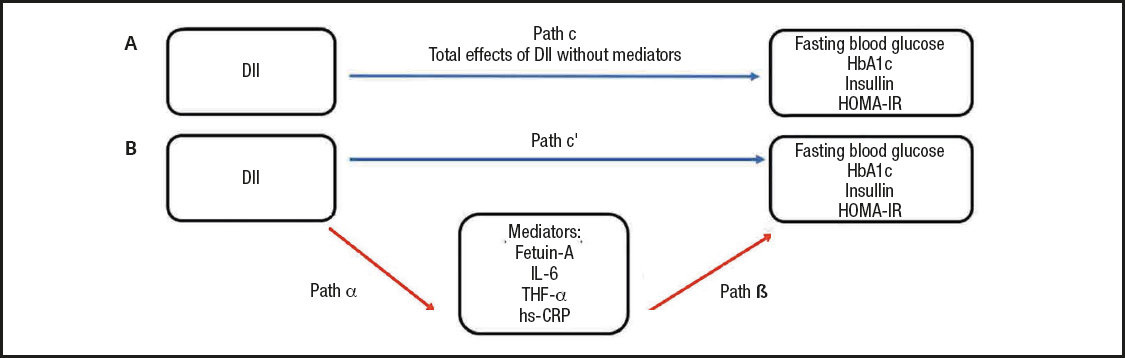
Figure 1. Model used in the simple mediation analysis of the association between the dietary inflammatory index (DII) and glucose metabolism markers, including fasting glucose, glycated hemoglobin (hbA1c), insulin, and homeostatic model assessment of insulin resistance (HOMA-IR) [adapted from Preacher and Hayes (33)]. Path "c" shows the total effect of DII on glucose metabolism markers without adjusting mediator variables (A); path α shows the regression coefficient between the DII and the mediator variables (b); path β, shows the regression coefficient between the mediator variables and glucose metabolism markers (B); path "c'" shows the ‘direct effect' of DII on glucose metabolism markers after adjusting the effect of the mediator variables (B). The product of regression coefficients α and β (αβ) shows the ‘indirect effect' (mediated effect) of DII on glucose metabolism markers via the mediator variables (B).
RESULTS
The baseline clinical data of the study population for the case and control groups are shown in table I. The average age of the case group was 43.5 ± 4.2 years, and that of the control group was 36.5 ± 5.7 years, with a statistically significant difference between groups (p < 0.05). A statistically significant difference was noted between the groups in terms of BMI (p < 0.05). However, there was no statistically significant difference between groups in the values of waist circumference, hip circumference, and waist-to-height ratio (p > 0.05). The median value of the DII scores of the case group was significantly higher than that of the control group (p < 0.05). The case group had significantly higher WBC, LYM, NEU/LYM, triglyceride, total cholesterol, LDL-cholesterol, and all glucose metabolism markers, including fasting blood glucose (FBG), insulin, HbA1c, and HOMA-IR values, in comparison to the control group (p < 0.05). Inflammatory markers including fetuin-A, IL-6, and TNF-α were also found to be significantly higher in the case group than in the control group (p < 0.05). Table II presents the relationship between DII score and energy, nutrients, and food component intake for both the case and control groups and the total study population. As shown in the table, there was a significant negative correlation between the DII score and dietary fiber, iron, vitamin A, β-carotene, vitamin E, vitamin B6, folic acid, flavones, and flavonols in total (p < 0.05). The relationship between the DII score and both glucose metabolism markers and inflammatory markers is displayed in table III. In the total study group, there was a significant positive correlation between DII and FBG, hbA1c, HOMA-IR, fetuin-A, IL-6, and hs-CRP, whereas a significant positive correlation was found between hbA1c, fetuin-A, and IL-6 in the case group, and IL-6 and hs-CRP in the control group. Table IV shows the ORs and 95 % CIs of the DII score and inflammatory markers for T2DM. After adjusting for potential confounding factors (age, physical activity, standardized energy intake, and BMI), the participants who consumed a high pro-inflammatory diet had a 2.0 times higher risk of developing T2DM (OR = 2.043; 95 % CI: 0.955, 4.371, p = 0.066). Considering the ORs of inflammatory markers, the participants who had higher levels of fetuin-A had a 1,2 times higher risk of developing T2DM (OR = 1.155; 95 % CI: 1.030, 1.296, p = 0.014). Likewise, the participants who had higher levels of IL-6 had 1.1 times (OR = 1.053; 95 % CI: 1.006, 1.102, p = 0.028), and those who had higher levels of TNF-α had a 7.2 times higher risk of developing T2DM (OR = 7.234; 95 % CI: 2.312, 22.631, p = 0.001). Table V presents the β-coefficients (95 % CIs) of the association between DII and glucose metabolism markers. A consistent finding was that IL-6 had a significant partial mediator role on the association between DII and glucose metabolism markers, including FBG [β = 10.574 (95 % CI: 3.705-17.443)], hbA1c [β = 0.469 (95 % CI: 0.174-0.764)] and HOMA-IR [β = 0.544 (95 % CI: 0.143-0.946)]. Both fetuin-A and hs-CRP had a significant full mediator role on the association between DII and HOMA-IR [respectively, β = 0.371 (95 % CI: -0.029-0.770), β = 0.424 (95 % CI: -0.007-0.856)]. The proportion of the effect mediated by fetuin-A was 35.2 %, whereas the proportion of the effect mediated by hs-CRP was 25.9 % (p < 0.05).
Table I. Baseline characteristics, biochemical measurements, and DII scores of the case and control groups
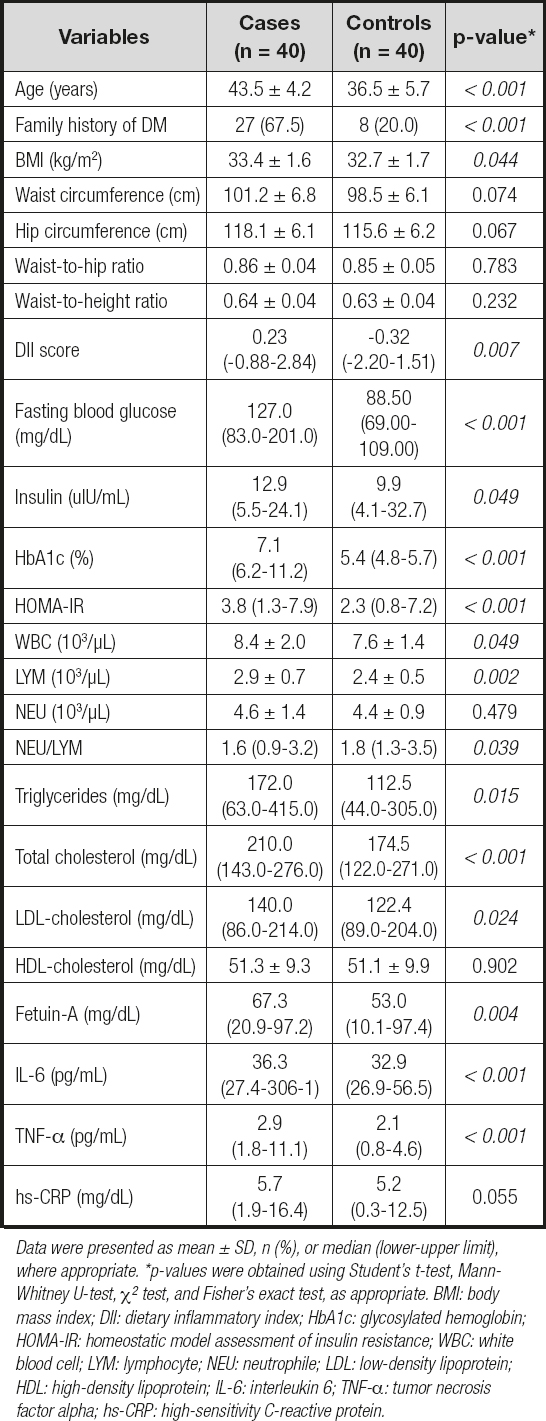
Data were presented as mean ± SD, n (%), or median (lower-upper limit), where appropriate. *p-values were obtained using Student's t-test, Mann-Whitney U-test, Χ2 test, and Fisher's exact test, as appropriate. BMI: body mass index; DII: dietary inflammatory index; HbA1c: glycosylated hemoglobin; HOMA-IR: homeostatic model assessment of insulin resistance; WBC: white blood cell; LYM: lymphocyte; NEU: neutrophile; LDL: low-density lipoprotein; HDL: high-density lipoprotein; IL-6: interleukin 6; TNF-α: tumor necrosis factor alpha; hs-CRP: high-sensitivity C-reactive protein.
Table II. Correlation analysis of DII with energy, nutrients, and dietary components between both the case and control groups, and the total study population
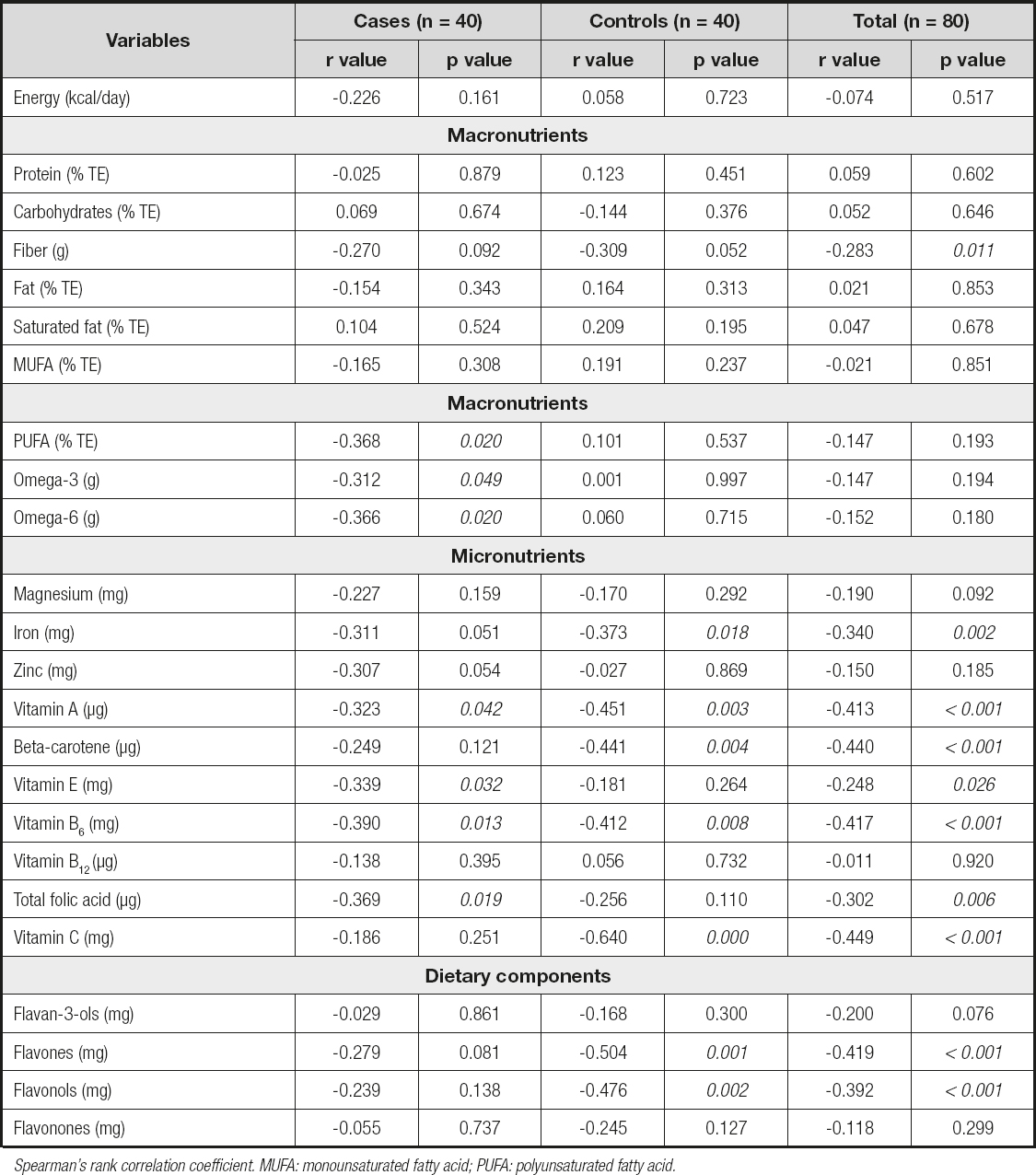
Spearman's rank correlation coefficient. MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid.
Table III. Correlation analysis of DII with glucose metabolism markers and inflammatory markers between both the case and control groups and the total study population

Spearman's rank correlation coefficient. HbA1c: glycosylated hemoglobin; HOMA-IR: homeostatic model assessment of insulin resistance; IL-6: interleukin 6; TNF-α:tumor necrosis factor alpha; hs-CRP: high-sensitivity C-reactive protein.
Table IV. Odds ratios (ORs) and 95 % confidence intervals (95 % CIs) for T2DM for DII score and inflammatory markers (n: 80)
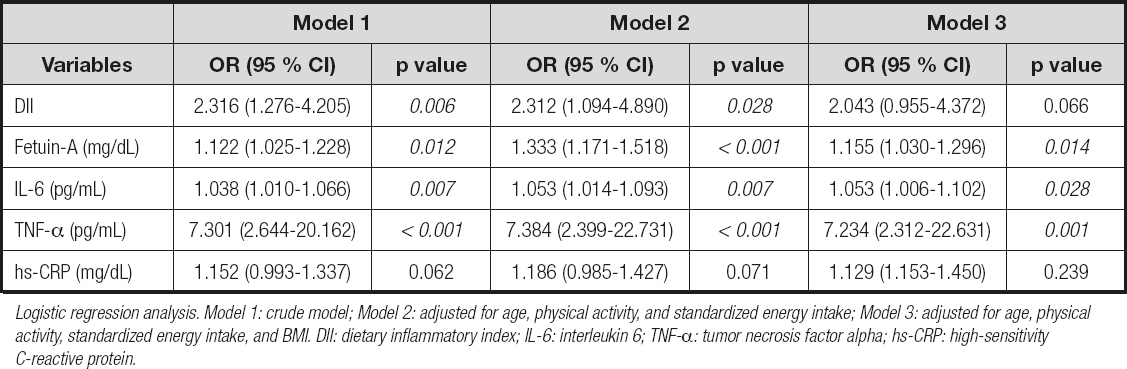
Logistic regression analysis. Model 1: crude model; Model 2: adjusted for age, physical activity, and standardized energy intake; Model 3: adjusted for age, physical activity, standardized energy intake, and BMI. DII: dietary inflammatory index; IL-6: interleukin 6; TNF-α: tumor necrosis factor alpha; hs-CRP: high-sensitivity C-reactive protein.
Table V. Total, direct and indirect effects of DII on glucose metabolism markers with inflammatory markers as mediators (n: 80)
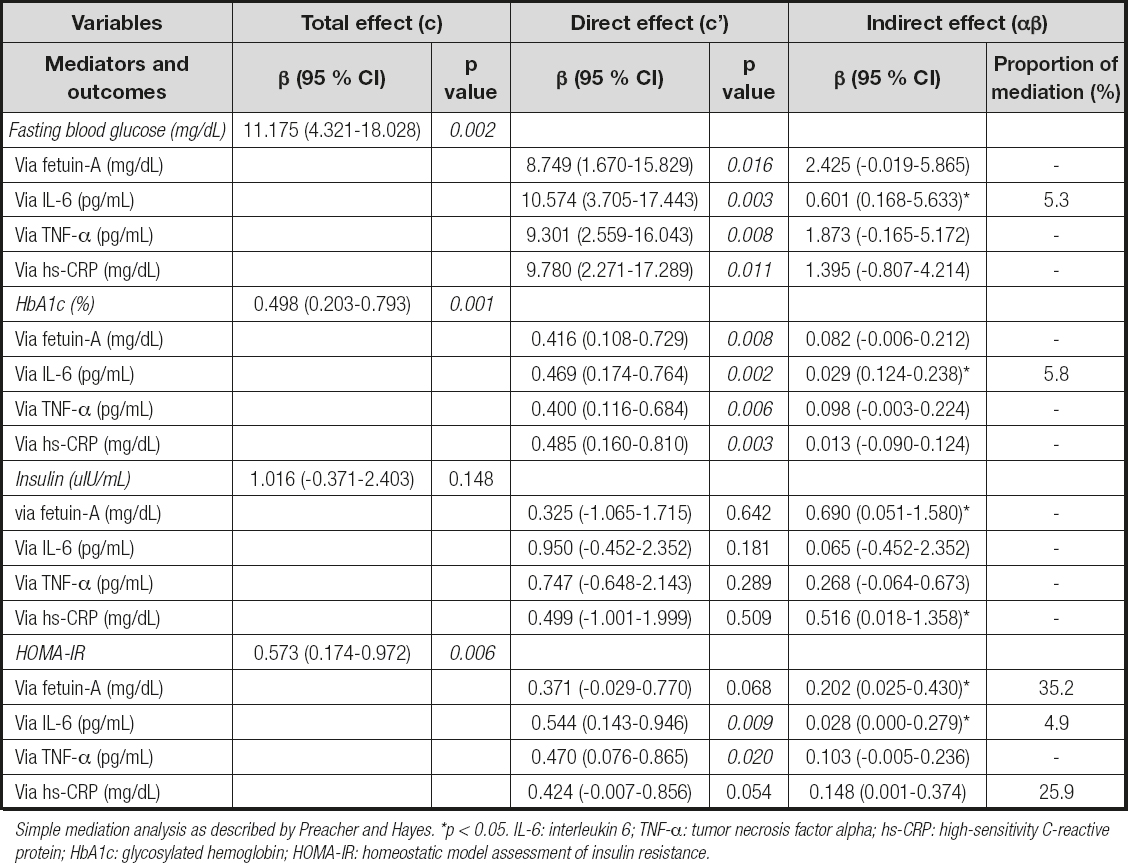
Simple mediation analysis as described by Preacher and Hayes. *p < 0.05. IL-6: interleukin 6; TNF-α: tumor necrosis factor alpha; hs-CRP: high-sensitivity C-reactive protein; HbA1c: glycosylated hemoglobin; HOMA-IR: homeostatic model assessment of insulin resistance.
DISCUSSION
In the present study, it was demonstrated that DII scores, which indicate the inflammatory potential of diet, were associated with inflammatory markers and T2DM risk, and that inflammatory markers including fetuin-A, IL-6, and TNF-α were also significantly associated with T2DM risk. In addition, the mediation analysis suggested that these relationships were fully mediated by fetuin-A and were also fully/partly mediated by hs-CRP and IL-6. To the best of our knowledge, this is the first study that investigated the mediator effects of fetuin-A on the association between diet and T2DM.
Studies in the past few decades have shown that inflammation may be one of the important factors in T2DM risk (20,21). It may be said that diet also affects T2DM by causing inflammation. Previous studies have indicated that a western diet, which represents a pro-inflammatory diet, is associated with a high risk of T2DM. In contrast, the Mediterranean diet, which represents an anti-inflammatory diet, has been associated with a low risk of T2DM (12-14). Recently, the DII has been used frequently in studies investigating the effect of the inflammatory load of diet on non-communicable diseases, including diabetes. Denova-Gutiérrez et al. (22) reported having a higher risk of diabetes in participants with high DII scores (who consume a more pro-inflammatory diet) than in those with low DII scores (who consume a more anti-inflammatory diet). In another study that investigated DII and diabetes risk, it was shown that the risk of diabetes increased by 13 % with every 1-point increase in DII score (17). The findings of the present study are in line with these studies, and it was found that the risk of diabetes also became higher as the DII scores of individuals increased.
The utility of the DII for assessing the inflammatory potential of the diet was shown in many studies (23,24). It was shown that individuals with high DII scores had a high consumption of pro-inflammatory nutrients, and individuals with low DII scores had a high consumption of nutrients with anti-inflammatory properties (15,22). Consistent with these findings, in the current study there was also a negative correlation between the DII score and anti-inflammatory nutrients such as MUFA, PUFA, n-3 and n-6 fatty acids, and antioxidant food components, as well as a positive correlation between the DII score and pro-inflammatory nutrients such as carbohydrates, total fat, and saturated fatty acids. Moreover, the DII has been constructed and was validated using inflammatory markers such as hs-CRP and IL-6 in the previous studies (23,24). The results of the present study are also in agreement with other studies in the literature where a significant association was reported between DII and inflammatory biomarkers.
In the current study, a relationship was found between all inflammatory markers and T2DM (hs-CRP was non-significant). Individuals with higher levels of fetuin-A, IL-6, TNF-α, and hs-CRP had a higher risk of developing T2DM. Proinflammatory cytokines that lead to inflammation cause β-cell damage and chronic hyperglycemia by affecting various pathways, and T2DM occurs as a result (25). Stefan et al. (5) demonstrated that fetuin-A was associated with the risk for incident diabetes, and was independently involved in the pathogenesis of T2DM. Their findings support that fetuin-A is a reliable predictor of T2DM. In addition, in many studies a positive correlation of TNF-α and IL-6 with T2DM was shown, and evidence that the pattern and variation of these pro-inflammatory cytokines are important in the pathogenesis of T2DM was provided (26).
The effects of diet on inflammation and inflammation on diabetes suggest that diet may also increase T2DM risk through inflammation (11). One of the possible mechanisms to explain the relationship between diet and T2DM may be that a pro-inflammatory diet causes insulin resistance via inflammatory markers. Diet is a crucial predictor of inflammatory marker levels in the circulation. An anti-inflammatory Mediterranean diet supplemented with virgin olive oil or nuts reduced serum C-reactive protein, IL-6, and endothelial and monocytic adhesion molecules and chemokines in 772 subjects at high risk for CVD (27). In contrast, a pro-inflammatory high-fat diet promotes inflammation (28). Unhealthy diets promote a pro-inflammatory setting marked by higher cytokine levels (IL-1β, IL-6, and TNF-α) and CRP. As a result, a prolonged pro-inflammatory state induced by diet could cause insulin resistance (29). In the present study, inflammatory markers including fetuin-A, IL-6 and hs-CRP had a fully/partly mediator role between DII, indicating dietary inflammatory potential, and HOMA-IR. These results supported the hypothesis that inflammation mediates the relationship between diet and insulin resistance. Van Woudenbergh et al. (11) also showed a significant mediating role of low-grade inflammation in the association between diet and insulin resistance. Conversely, in a study with a black South African population, the mediating effect of inflammation was not found in the association between diet and HOMA-IR and other glucose metabolism markers; however, adiposity had a mediator role (14). It might be that the direct effect of inflammation might be diminished in the presence of obesity, the most detrimental risk factor. Besides, ethnicity may modify the effect of risk factors on insulin resistance (30). In addition, since the inflammation mechanism of action is mediated by paracrine and autocrine effects, the specific effects of inflammation may not be entirely determined (14).
Notably, fetuin-A explained the highest significant proportion of the association between DII and HOMA-IR. The fact that fetuin-A is an endogenous inhibitor of the insulin receptor tyrosine kinase is a major factor in this result (6). It has been shown that fetuin-A inhibits insulin signaling by inhibiting insulin receptor tyrosine kinase in skeletal muscle and hepatocytes, and causes insulin resistance in these target tissues in vitro (31,32). In addition, Stefan et al. (33) demonstrated that fetuin-A in rodents inhibited insulin receptor tyrosine kinase phosphorylation. Moreover, human studies showing the relationship between serum fetuin-A and insulin resistance are limited, although increasingly common as of today (7,34,35).
The inability to infer cause-effect relationships due to its design may be considered the main limitation of this study. Besides, the small sample size may have affected the statistical power to identify some effects. In addition, the study population consists exclusively of women. Therefore, the results should be verified by future prospective longitudinal studies with larger sample sizes including both genders. However, there are several strengths of the present study. First, this is the first study investigating the mediator effects of fetuin-A in the association between diet and T2DM. Because the participants were women, an age limitation was applied due to the unknown effects of the menstrual cycle on fetuin-A and to eliminate the effects of the physiological changes related to menopause. In addition, BMI inclusion criteria were kept within narrow ranges in terms of study homogeneity. Finally, although the FFQ may cause measurement errors even in healthy individuals, it provided access to 44 of the 45 food parameters required to calculate the DII. Many previous studies used fewer parameters (17,22).
In conclusion, these findings suggest that a pro-inflammatory diet, by creating an environment of increased inflammatory markers, may affect glucose metabolism, in particular insulin resistance, through these markers and ultimately cause T2DM. In addition, fetuin-A may also act as an important novel mediator between diet and T2DM by inducing insulin resistance. These results strengthen the fact that diet has an important role in developing T2DM risk in obese women. Considering these findings, adopting an anti-inflammatory diet approach may be a helpful strategy for preventing insulin resistance and reducing risk of diabetes. Further prospective longitudinal studies with a larger sample size are needed to investigate the effects of fetuin-A on insulin resistance, and its mediating role in diet and T2DM. In these studies, the molecular mechanism of fetuin-A may be examined, and whether fetuin-A may be used as a clinical marker in T2DM may also be tested.














