INTRODUCTION
Vitamin D is obtained from dietary sources and produced in the skin. As is well known, vitamin D plays an essential role in calcium absorption and bone health. Its deficiency has been associated with autoimmune, cardiovascular and muscular diseases and other chronic conditions (1).
Hypovitaminosis D -assessed by serum concentration of 25-hydroxyvitamin D [25(OH)D], a widely accepted biomarker of vitamin D status (2)- has been found to be common in the general population and is even more prevalent in patients with chronic kidney disease (CKD) undergoing haemodialysis (HD), with an incidence of up to 90 % (1). According to several studies carried out in Spain, hypovitaminosis D is more prevalent in the population with CKD on HD compared to the healthy population (69 %-78 % vs. 30 %-44 %, respectively) (3).
An inadequate nutritional status due to dietary restrictions and lack of appetite due to a uraemic environment can trigger nutritional deficiencies in HD patients, altering serum concentrations of vitamin D (4). However, many other factors such as insufficient sunlight exposure, aging and impaired renal synthesis can also reduce levels of 25(OH)D in patients with chronic kidney disease (CKD) (1,5). Its deficiency can lead to several consequences in HD patients: secondary hyperparathyroidism, high levels of bone turnover markers, low bone mineral density, muscle weakness, increased risk of falls, metabolic syndrome, insulin resistance, cognitive impairment, and progression of kidney disease, among others (1). It is also associated with increased mortality in HD patients (6,7). These outcomes will in turn worsen the patient's nutritional status and quality of life (QoL).
In this sense, the purpose of this cross-sectional study is to assess the prevalence of hypovitaminosis D in patients undergoing HD and to determine its relationship with nutritional status and QoL.
MATERIALS AND METHODS
STUDY POPULATION
The study population consisted of 120 patients receiving periodic HD. Those who agreed to participate and met the inclusion criteria were enrolled in a consecutive manner. Data for these patients were collected by non-purposive sampling, from January 2017 to December 2017, and were retrospectively analysed. The following inclusion criteria were applied: adults (aged at least 18 years) who had not previously consulted with a dietician and who had received the HD programme, uninterruptedly, for at least three months. The HD sessions were held three times per week for four hours. No participants were lost to follow-up.
STUDY DESIGN
The study was coordinated by the Nephrology and Pharmacy & Nutrition Departments of the Costa del Sol Hospital, Marbella (Spain), with the participation of specialists in nephrology, nursing and clinical nutrition. Ethical approval was granted by the Costa del Sol Research Ethics Committee on May 30, 2019 with approval number 85-05-2019. The ethical principles set forth in the most recent version of the Declaration of Helsinki and the standards of good clinical practice were all adhered to.
MALNUTRITION INFLAMMATION SCORE
The malnutrition inflammation score (MIS) was employed to determine the risk of malnutrition, from which a nutritional diagnosis was made, categorising patients as normal nutritional status, at risk of malnutrition or malnourished.
Various methods can be used to assess nutritional status in dialysis patients, and there is no single gold standard. The MIS, described by Kalantar et al. (8), uses some elements of the Subjective Global Assessment (SGA) (9) and includes comorbidity according to the duration of dialysis treatment, together with biochemical parameters such as albumin and transferrin. The MIS has four sections: nutritional history, physical examination, body mass index and laboratory values. The final score possible ranges from 0 to 30 points, and scores > 5 indicate the presence of nutritional risk (NR). MIS is widely used in CKD (10,11) and is supported by studies which have demonstrated its value as a predictor of mortality, morbidity and protein-energy wasting syndrome (12-15).
In the present study, a nutritional diagnosis was made according to the consensus on malnutrition established by the Spanish Society of Enteral and Parenteral Nutrition and the Spanish Society of Medical Documentation (16), according to which malnutrition is classified into three groups: protein malnutrition, caloric malnutrition or mixed malnutrition (caloric-protein). The latter classification is further divided according to the severity of malnutrition, as mild, moderate or severe.
KIDNEY DISEASE QUALITY OF LIFE QUESTIONNAIRE
QoL was measured by the validated Spanish version of the Kidney Disease Quality of Life questionnaire version 1.2 (KDQOL-SF™) (17). This instrument includes 43 specific items for patients with kidney disease, organised into 11 specific dimensions: symptoms/problems, effects of kidney disease, burden of kidney disease, work status, cognitive function, quality of social interaction, sexual function, sleep, social support, dialysis staff encouragement and patient satisfaction. The KDQOL-SF also has a generic part that includes the 36 items of the SF-36 questionnaire. It is composed of eight dimensions and provides two summary scores, for physical and mental health, and reports levels of physical functioning, physical role, pain, general health, emotional well-being, emotional role, social function and energy/fatigue. The two summary scores are the physical component summary (PCS) and the mental component summary (MCS).
Each question is numerically coded and then transformed into a scale from 0 to 100, on which higher values reflect a better QoL. It also includes an item for health status, measured on a scale from 0 to 10, where 0 indicates “the worst possible health (as bad as or worse than being dead)” and 10 indicates “the best possible health”.
PHYSICAL FUNCTION
The Barthel index measures the extent to which somebody can function independently and has mobility in their activities of daily living, indicating the level of functional capacity (18). The total score possible ranges from 0 to 100, with lower scores suggesting increased disability.
LABORATORY EVALUATIONS AND UREA KINETIC MODELLING
Blood measurements were taken in the morning after an 8-h overnight fast. Blood samples consisted of electrolyte assay (phosphorus, potassium, sodium), biochemical parameters (haemoglobin, creatinine, glomerular filtration rate, glucose, cholesterol, total proteins, albumin, transferrin, prealbumin, C-reactive protein and glycated haemoglobin), assessed by standard laboratory methods, and hormone assessment (parathyroid hormone and 25(OH)D).
The best indicator of vitamin D concentration is the serum level of 25(OH)D (2), determined by chemiluminescence radioimmunoassay (Liasion®; Diasorin, Saluggia, Italy). In our study, the intra- and inter-assay coefficients of variation were 4.8 % and 7.8 %, respectively. According to KDIGO guidelines (19), there is no established cutoff point to define “insufficiency” or “deficiency” of 25(OH)D in CKD patients. For the purposes of our study, the following classification was used to define 25(OH)D serum concentrations: Sufficiency (≥ 30 ng/ml), Insufficiency (29-10 ng/ml) and Deficiency (< 10 ng/ml). Insufficiency and deficiency were assumed to be representative of hypovitaminosis D.
Single-pool Kt/V was measured in all patients as an indicator of dialysis adequacy, using the Daugirdas second generation formula (20). Data were obtained using the BBraun Dialog+® dialysis machine.
STATISTICAL METHODS
A descriptive analysis was performed of the total sample using measures of central tendency and dispersion for the quantitative variables, and of frequency distribution for the qualitative ones. The Pearson correlation coefficient was used to evaluate differences in the distribution of 25(OH)D.
Multiple linear regression analysis was performed to determine how each sociodemographic and clinical characteristic influences QOL. The outcome variables were PCS, MCS and the Kidney Disease Component Summary (KDCS). Backward stepwise selection was used for the independent variables, with an entry criterion of p < 0.05 and an exit criterion of p > 0.1. β-coefficients were calculated, with their respective 95 % confidence intervals (CIs). Multiple logistic regression analysis was performed to estimate the odds ratios (OR) and 95 % CIs, which were used to determine the relationship between hypovitaminosis D and malnutrition.
The level of statistical significance was set at p < 0.05. Data were analysed using the SPSS statistical software package, version 28.0.
RESULTS
A total of 120 HD patients were included in the study. The mean age of the participants was 68 ± 16 years, of whom 67 % were male. Hypovitaminosis D was detected in 71 % of these patients; 38 % presented 25-hydroxyvitamin D levels corresponding to a deficiency status, 33 % presented an insufficient status and only 29 % of patients presented levels within the normal range. According to the Barthel index, 25(OH)D deficiency is correlated with greater dependence (p < 0.001). In our sample, 89 % of the patients with hypovitaminosis D were dependent for the basic activities of daily living.
Diabetes mellitus was present in 46 % of the participants and the incidence of diabetic nephropathy aetiology increased with the severity of the hypovitaminosis. The sociodemographic, clinical and biochemical characteristics of the study population, with respect to 25-(OH)D levels, are detailed in table I.
Table I. Sociodemographic and clinical characteristics of patients included in the study according to serum 25(OH)D concentration groups.
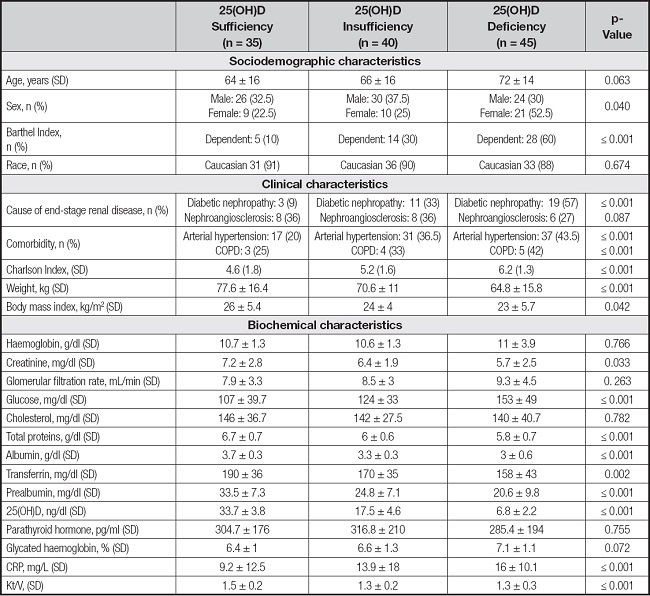
Data are expressed as interquartile range (IQR) and percentage (%).
CRP: C-reactive protein; COPD: chronic obstructive pulmonary disease.
For the total population, NR was assessed using the MIS scale. NR was associated with lower levels of vitamin D; thus, among the 62 % of the total population at NR (MIS > 5), 92 % had hypovitaminosis D. Patients with sufficient levels of vitamin D had a mean MIS score of 4.3 ± 1.3, presenting no NR, while those presenting insufficiency or deficiency had mean MIS scores of 7 ± 3.2 and 8.6 ± 2.5, respectively, and were considered to be at nutritional risk (< 0.001).
After the initial screening, all patients received a nutritional education programme consisting of a personalised diet and nutritional recommendations, based on an individualised nutritional assessment. This procedure led the study population to be divided into two main groups, well-nourished and malnourished. The latter were subsequently classified as presenting mild, moderate or severe protein-energy malnutrition and protein malnutrition.
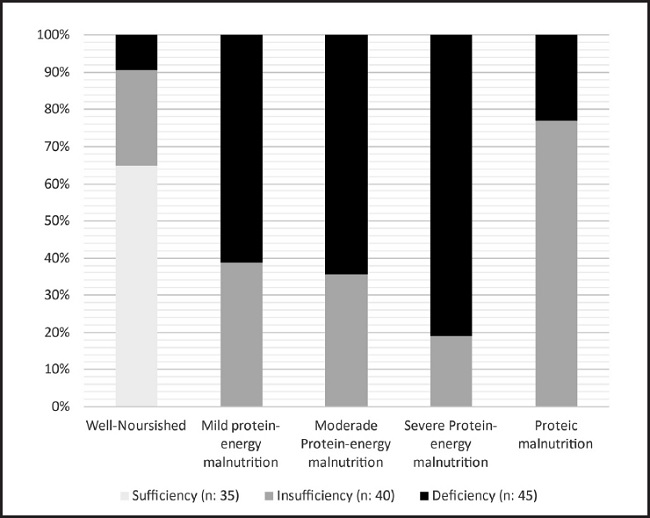
After categorising the nutritional diagnoses according to the serum 25(OH)D concentration groups identified, a directly proportional correlation was observed between malnourishment and hypovitaminosis D. In all, 75 % of the patients with hypovitaminosis D presented malnutrition (n = 66), this condition being more prevalent (60 %) among those who were deficient in 25(OH)D (n = 40), p < 0.001. On the other hand, the patients whose vitamin D levels were normal were all classed as well-nourished (Fig. 1).
QoL was assessed according to the 25(OH)D levels recorded. The results of the general summary areas are shown in table II. In the specific section of the questionnaire, the symptoms/problems, effects and the burden of kidney disease (p < 0.001) and work status (p < 0.05) scores all decreased as 25(OH)D worsened. In the SF-36 section, all areas were less favourable in the patients with 25(OH)D deficiency than in those with optimal values (p < 0.001).
Table II. Component summary scores according to serum 25(OH)D concentration groups.

Data are expressed as mean ± standard deviation for normally-distributed data.
Multiple regression analysis was used to investigate the relationship between sociodemographic and clinical variables, with the general summary areas of QoL as response variables (Table III). Five explanatory variables were included: the Charlson and Barthel Indexes, sex and the two subgroups of hypovitaminosis D: insufficiency and deficiency.
Table III. Multiple linear regression analysis.

Data are expressed as mean ± standard deviation for normal data.
KDCS: Kidney Disease Component Summary; PCS: Physical Component Summary; MCS: Mental Component Summary.
The 25(OH)D deficiency group presented the highest level of explanation on the KDCS (ß = -8.11, 95 % CI: -12.6 to -3.5, p < 0.001), explaining 21 % of the variance adjusted by the Charlson index; on the PCS (ß = -13.7, 95 % CI: -19.4 to -8.0, p < 0.001), explaining 27 % of the variance adjusted by the Barthel index; and on the MCS (ß = -9.45, 95 % CI: -13.6 to -5.2, p < 0.001), explaining 22 % of the variance, adjusted by sex.
With independent adjustment for malnutrition, multivariate logistic regression was performed, showing that vitamin D deficiency provided the highest level of explanation for malnutrition, accounting for 65 % of the variance (OR = 14.6, 95 % CI: 5.0 to 41.1, p < 0.001).
DISCUSSION
According to the results obtained, HD patients presented a high rate of hypovitaminosis D. These findings are in agreement with those obtained in previous studies (21,22). For example, one study of a Spanish population reported serum levels of 25(OH)D below 30 ng/ml among 94 % of HD patients (3). In our study, 71 % of the population presented hypovitaminosis D.
This outcome may arise because with increasing age, physical activity decreases, as does exposure to ultraviolet B radiation. Moreover, the impaired expression of renal 1-α-hydroxylase, caused by a reduction in the nephron mass, reduces the concentration of its active form 1,25(OH)2D. A restrictive diet may also produce nutritional vitamin D deficiency. All of these factors are correlated with hypovitaminosis D in patients with CKD (1,23).
Although vitamin D is traditionally associated with bone and mineral metabolism, its deficiency in CKD patients is also correlated with a wide variety of illnesses and conditions, such as secondary hyperparathyroidism, metabolic syndrome (obesity, insulin resistance and diabetes mellitus), progression of kidney disease, cardiovascular disease, autoimmune and neurodegenerative diseases, cognitive impairment, depression, vascular calcification, arterial stiffness, cancer, risk of falls and fractures, sarcopenia and greater mortality (1). It has been reported that in patients with advanced CKD, including HD patients, higher 25(OH) vitamin D levels are associated with significantly improved survival. Indeed, one meta-analysis suggesting that for each 10 ng/ml increase in 25(OH) vitamin D levels, there is a 14 % lower relative risk of death [0.86 (95 % CI, 0.82-0.91)]. Therefore, it appears that higher levels would be associated with better outcomes (24). Accordingly, patients are likely to have a poorer nutritional status and impaired QoL. For this reason, we specifically investigated these factors, according to the 25(OH)D levels assessed by MIS and the KDQOL-SF™ questionnaire results.
To the best of our knowledge, no previous studies have been conducted to assess the value of 25(OH)D as a marker of nutritional risk, using the MIS nutritional screening instrument. According to our study results, there is a strong association between vitamin D deficiency and NR; thus, of the 62 % of the total population at NR (MIS > 5), 92 % had hypovitaminosis D. Olvera et al. (25) used the SGA screening tool to assess nutritional status in patients with stage 4 CKD according to serum 25(OH)D concentrations, and reported a prevalence of 16.7 % of severe malnutrition in patients with 25(OH)D levels < 10 ng/ml. However, the differences between the study groups were not statistically significant.
To date, there is still insufficient study evidence on the association between 25(OH)D and nutritional status. Our results show that 55 % of the total study population were malnourished, and that of these 75 % presented concentrations of 25(OH)D below 30 ng/ml, with p < 0.001. However, Bednarek-Skublewska et al. (26) found no association between vitamin D levels and nutritional status.
Our study findings show that patients with vitamin D deficiency have a lower average QoL. In this respect, Oh et al. (27) studied a cohort of non-dialysis patients with stage 1-5 CKD, using the same questionnaire tool, and also recorded significantly lower KDCS, PCS and MCS scores in the vitamin D deficiency group than in patients with no such deficiency.
Yuksel et al. (28) and Kara et al. (29), too, have studied the QoL in dialysed patients, but using another questionnaire tool, KDQOL-36. Nevertheless, both questionnaires are based on the generic 36-item health survey and obtain scores in general areas like the PCS and MCS. The results obtained were comparable with ours, i.e. both studies revealed significantly worse scores in the above respects for patients with 25(OH)D deficiency. These findings are consistent with those reported in other research concerning the role of vitamin-D in the musculoskeletal system (30) and the central nervous system (31), suggesting that dialysed patients perform less physical activity and may present impaired cognitive function.
The following limitations to our study are acknowledged. Firstly, its cross-sectional nature means that causality cannot be established between serum 25(OH)D concentrations, nutritional status and QoL in dialysed patients. Moreover, factors influencing the synthesis of vitamin D in the skin, such as the dietary consumption of vitamin D or the duration of exposure to sunlight, were not assessed.
Both the KDOQI (32) and KDIGO (2) experts recommend checking and supplementing low serum 25(OH)D levels in patients with CKD on HD. However, little is known about the importance of an early nutritional approach through a nutritional education program to address this issue. Therefore, one strength of this study is the fact that it implemented a protocol in which patients received education and informational material to promote consumption of foods rich in vitamin D, making them aware of its importance and repercussions on health. The results of the efficacy of this early nutritional intervention protocol in the prevention of hypovitaminosis D and its relationship to nutritional status and QoL in HD patients will be fully described in subsequent scientific articles.
In short, this study shows that hypovitaminosis D is frequently experienced by HD patients. Lower 25(OH)D levels are associated with greater NR, poorer nutritional status and worse QoL. In view of these findings, new strategies, such as nutritional advice on the appropriate intake of vitamin D or controlled vitamin D supplementation, should be adopted. Further long-term observation is also required to assess the potential effects of vitamin D on HD patients.