INTRODUCTION
Enteral nutrition (EN) is the preferred feeding route for critically ill patients on mechanical ventilation (MV) (1), and it is associated with better immunological and clinical outcomes when started within 48 hours after admission (2). EN interruption is common during ICU stay due to procedural-related fasting, vasopressors doses, and other potentially avoidable causes (3). Feeding intolerance (FI), defined as gastrointestinal (GI) dysfunction resulting in a reduction in the delivery of enteral feeding, regardless of the underlying cause, is another common cause of EN interruption (4). Different definitions of GI symptoms are currently used in studies (5), and include vomiting, regurgitation, abdominal distension, gastric residual volume, absent/abnormal bowel sounds, abdominal pain, absence/presence of stool, diarrhea, high intra-abdominal pressure and GI bleeding (6).
Prone positioning (PP) is commonly used in acute respiratory distress syndrome (ARDS) patients (7,8), and may be a cause of EN interruptions due to higher episodes of vomiting and gastric residual volume (GRV) (9), intra-abdominal hypertension (IAH), and gut dysmotility, which can also be exacerbated by pharmacotherapy (7,10,11). Furthermore, the incidence of ARDS is higher in obese patients, and could be beneficiated by PP (12,13). Since obesity has been associated with a higher risk of impaired gastric motility and EN intolerance, due to IAH (14,15) and comorbidities such as diabetes (16), there is a lack of knowledge about the safety of EN provision in overweight and obese patients in PP. The aim of this study is to compare the incidence of FI during the supine or prone position in overweight and obese patients on MV.
METHODS
This was a retrospective cohort of consecutive patients that were admitted to the Intensive Care Unit (ICU) from November 2020 to December 2021. COVID-19 patients (confirmed by SARS-CoV-2 real-time polymerase chain reaction) on MV due to ARDS were included (17). Patients were categorized as PP if they required this management during the first five days of EN, or supine positioning (SP) if not required during that same period. PP was considered when PaO2/FiO2 was below 150 according to current treatment guidelines (18). Patients were excluded if they did not have overweight/obesity (body mass index [BMI] < 25 kg/m2), or if their management required either MV or PP for less than five days, or position changed during the same period. This study was reviewed and approved by the Institutional Review Board (Register #C51-29).
DATA COLLECTION
Demographic information including sex and age was collected from patient records. Prescribed drugs with nutritional implications (steroids, benzodiazepines, opioids, neuromuscular blocking agents [NBA], sedatives, dexmedetomidine, prokinetics and vasopressors) along with medical history of non-communicable diseases were also registered. According to our unit standard, PP was achieved with foam wedges and pillows, alternating head, and neck rotation every four hours. Duration of PP cycle is continuous (24 hours) without cycles SP changed.
NUTRITIONAL ASSESSMENT
Nutritional assessment was performed on all patients during the first 24-48 hours after ICU admission. Body weight and height were estimated by means of validated equations using anthropometric measurements (19). BMI was calculated and classified using the World Health Organization (WHO) criteria (20). Waist circumference was measured at the midpoint of the line between the rib or costal margin and the iliac crest; measurements were made using a tape graduated in centimeters with 0.1 cm precision (SECA® 201, Germany). Body composition was assessed by bioelectrical impedance (BIA) using a multi-frequency device (InBody S10®, InBody Co., Ltd., Seoul, Korea). Fat mass in kg and phase angle were recorded from the machine output. The nutritional risk of each patient was calculated using a modified NUTRIC-Score due to the unavailability of the IL-6 determinations. High nutritional risk was established with a score ≥ 5 (21).
ENTERAL NUTRITION PRESCRIPTION
Orogastric tube placement was used over other short-term accesses as a common institutional practice to avoid epistaxis and sinus infection. Following radiographic confirmation of the correct tip position, EN was initiated. Calories and protein were prescribed according to recommendations by the American Society of Parenteral and Enteral Nutrition (ASPEN) and European Society for Clinical Nutrition and Metabolism (ESPEN), with a general target of 25 kcal/kg and 1.3 g/kg, respectively (22,23). Adjusted body weight was used in patients with a BMI > 30 kg/m2 and was calculated using Hamwi equations. An algorithm for EN progression was used: 10 kcal/kg, 15 kcal/kg, and 20 kcal/kg on days 1, 2 and 3, respectively. Next, 25 kcal/kg on day 4 was prescribed in patients with high nutritional risk, and 20 kcal/kg during the first 4-7 days on low nutritional risk. Calories derived from non-nutritional sources were factored into the nutrition prescriptions to avoid overfeeding. In case of FI and interruption during EN advancement, prokinetics were administered and same nutritional support prescription from the previous day was maintained.
MAIN OUTCOME DEFINITION
FI incidence was defined as the presence of any of the following clinical conditions: diarrhea (frequency of bowel movement > 3 times/day Bristol 6 or liquid stools), vomiting, constipation (no evacuation during five consecutive days) and gastroparesis (gastric residual volume ≥ 200 and ≥ 500 ml, because there are common cut-off values used in hospital setting) (24,25). GRV was measured one time at day before EN initiation by aspirating the orogastric tube with a 50-ml syringe.
STATISTICAL ANALYSIS
Data were analyzed using Stata Intercooled (version 14, Stata Corporation, College Station, TX, USA). The normality of the distribution of quantitative variables was verified by the Shapiro-Wilk test. Descriptive statistics were used for the analysis of categorical variables (absolute and relative frequency) and quantitative variables (mean and standard deviation [SD] or median and interquartile range [IQR]). Clinical data between PP and SP were compared using the Student's t-test, Mann-Whitney U test or the χ2 test. Statistical significance was defined as p < 0.05. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.
RESULTS
A total of 180 patients were admitted to the ICU. Only 82 patients were included in this analysis (mean age ± SD: 45.3 ± 12.1; 70 % males) (Fig. 1). Overweight was identified in 59 % and obesity in 41 % according to BMI, with higher fat mass (%) in PP males (35 ± 10.8 vs 29.1 ± 8.9, p = 0.02) but not in females.
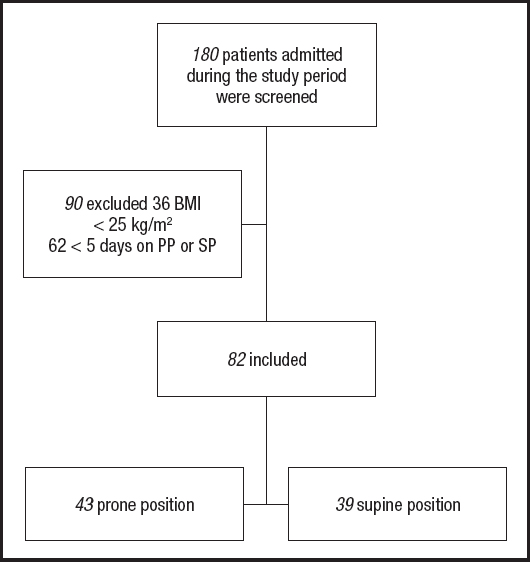
Figure 1. Patients included in this analysis. BMI: body mass index; PP: prone position; SP: supine position.
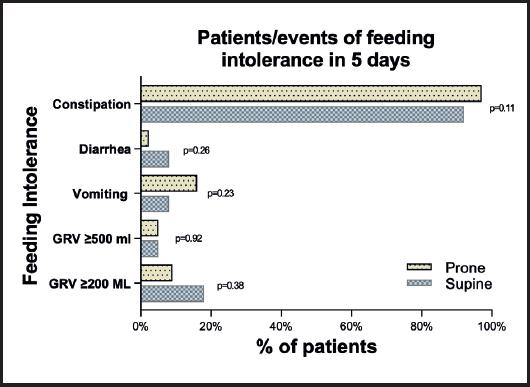
Statistical toward of low phase angle was observed in PP males (6.2 ± 1.4 vs 5.6 ± 0.9, p = 0.06), but not in females (Table I). Differences in FI were reported in table II; constipation was common in both groups (92 % vs 97 %, p = 0.11). GRV ≥ 200 (18 % vs 12 %, p = 0.38) and ≥ 500 ml (5 % vs 5 %, p = 0.92), vomiting (8 % vs 16 %, p = 0.23) and diarrhea events (8 % vs 2.3 %, p = 0.26) were similar in SP and PP groups (Fig. 2).
Table I. Demographical characteristics of critically ill COVID-19 patients.
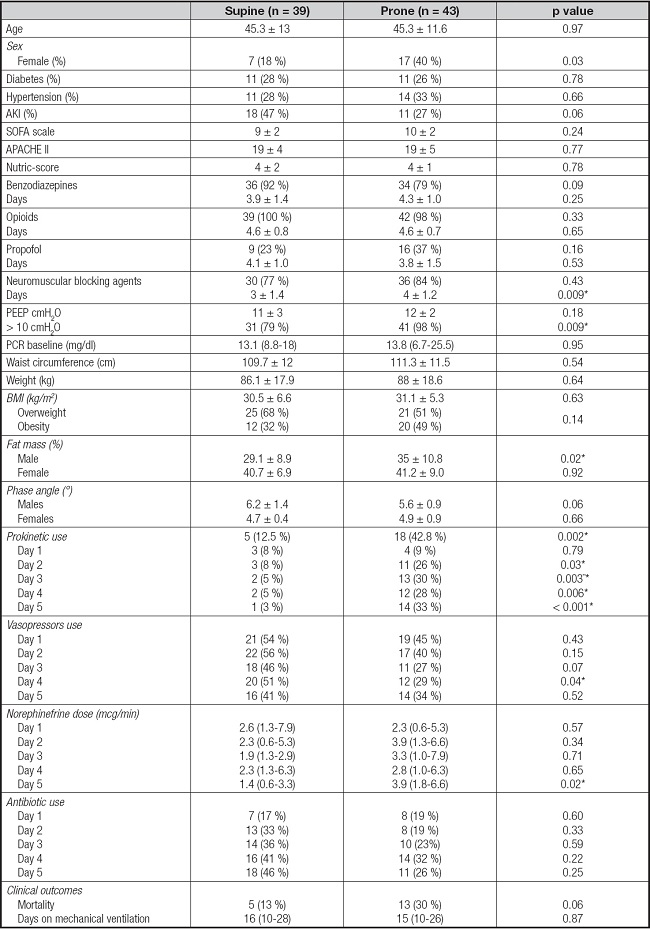
AKI: Acute Kidney Injury; SOFA: Sequential Organ Failure Assessment; APACHE: Acute Physiology and Chronic Health Evaluation II; PEEP: positive end-expiratory pressure; BMI: body mass index.
Table II. Differences in feeding intolerance according to prone or supine positioning.
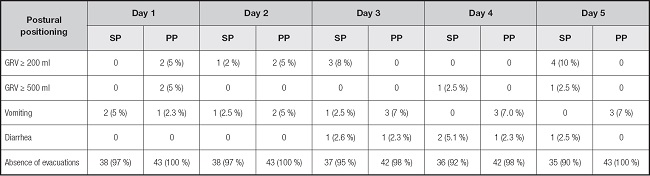
n (%). SP: supine position; PP: prone position; GRV: gastric residual volume.
DISCUSSION
This study found no differences in FI incidence between PP and SP patients on MV due to COVID-19 ARDS. In this cohort we included only patients with BMI ≥ 25 kg/m2 (59 % overweight and 41 % obesity) receiving EN in PP o SP continuously during the first five days of MV. No differences in BMI (p = 0.63) and phase angle by sex were observed between individuals at the SP or PP. Similar prevalence of overweight and obesity were observed by Savio R et al. in critically-ill patients with non-COVID related ARDS (49.2 % and 30.2 %, respectively) (26). Both studies showed no significant differences in FI despite SP or PP. This suggests that EN during PP is safe and does not represent a higher risk for GI dysfunction in overweight or obese individuals (27).
The use of certain drugs such as sedatives, opioids and vasopressors has also been related to FI (7). In our cohort, we found no differences in the use of benzodiazepines (p = 0.09), opioids (p = 0.33), propofol (p = 0.16) or antibiotics in SP compared to PP during the study period. The number of individuals receiving vasopressors in the SP group and the PP group was also similar, except for day 4 (51 % vs 29 %, p = 0.04); however, no significant differences were observed in FI events. Individuals in PP do not seem to have different or higher drug requirements compared to SP, which can be related to the fact that both groups reported a similar incidence of FI.
According to the guidelines and practice recommendations for nutritional care of critically-ill patients with COVID-19, it has been established that prokinetics use can be considered to promote gastric motility in high risk aspiration patients (28,29). In a systematic review from Lewis et al. prokinetics were associated with reduced FI; defined as GRV ≥ 150 ml, vomiting or abdominal distention (RR 0.73, 95 % CI: 0.55-0.97; p = 0.03), and high gastric residual volumes reduction (RR 0.69; 95 % CI: 0.52-0.91; p = 0.009). Nevertheless, there was no significant improvement in the risk of vomiting, diarrhea, ICU length of stay or mortality (30). In a recent meta-analysis from Peng et al. an updated definition of FI (GRV ≥ 500 ml, presence of vomiting, diarrhea, gastrointestinal bleeding or inadequate delivery of EN < 60 % of the target or < 20 kcal/kg after 72 hours of feeding attempts) was proposed. According to this, ten of 13 studies showed that prokinetics were beneficial on FI in critically-ill patients with gastric EN, with a reduction in ICU length of stay (MD -2.03, 95 % CI: -3.96, -0.10; p = 0.04; low certainty) and hospital length of stay (MD -3.21, 95 % CI: -5.35, -1.06; p = 0.003; low certainty) (31). However, prokinetics effect on other clinical outcomes related to ICU mortality such as pneumonia, remains unclear.
In our cohort, prokinetics were used routinely by some members of physician's staff in continuous PP as prevention or initiated after FI episodes; we observed that a higher frequency of individuals in our PP group received prokinetics, compared to the SP group (42.8 % vs 12.5 %, p = 0.002). In our cohort, constipation was common in both groups (97 % vs 92 %, p = 0.11). GRV ≥ 200, vomiting and diarrhea events were similar in SP and PP groups. Considering this, the initiation of prokinetics agents should be considered simultaneously at PP (7), to avoid fasting prescription previous to PP or the reduction of the rate infusion of EN as strategies for FI prevention.
Higher prevalence of obesity grade 1 (BMI ≥ 35 kg/m2) was observed in males on PP group (31 % vs 13 %, p = 0.26). This explain the higher amount of adipose tissue in males between groups. Obese patients have an increased risk of ARDS and were the group of patients that may benefit from PP. No differences were observed for phase angle between groups, which is a marker of low muscle mass and is associated with higher mortality and length of stay (32-34).
Similar to our results, other authors report no differences between positions in GRV and vomiting episodes in non-COVID-19 critically-ill patients with ARDS (26), however, BMI was not reported (35,36). Reignier et al. report that patients on PP and nasogastric tube had a higher GRV and had more vomiting episodes, however, nutritional status and BMI was not reported (37). Similarly, L'Her et al. report an incidence of high GRV in 14 % and vomiting in 12 % of patients on PP, but enteral access and BMI were not reported (38). Contrary to what can be expected, Liu et al. report that BMI ≥ 40 kg/m2 is associated with decreased risk of FI, but differences between PP and SP were not analyzed. The incidence rate variation between reports may be influenced by PP, BMI and enteral access (39).
Our study offers data in COVID-19 critically-ill patients with overweight and obesity, showing that EN during PP is not different from SP and might be considered as a safe intervention, but it also has the following limitations: a) our cohort was enrolled at a single center; d) no data of interruptions or caloric debt associated with reported FI during prone were available; c) no data of the timing of the initiation of prokinetics agents as prophylactic or treatment of FI was gathered, and this may have an impact on FI rate; d) infectious complications or the incidence of ventilator-associated pneumonia (VAP) were not assessed; and e) although the follow-up period in our study was limited to the first five days of MV, it includes the time of initiation and advancement of EN, where the deficit in its supply due to FI interruptions has been associated with adverse clinical outcomes.