INTRODUCTION
Diabetes mellitus (DM) is a chronic disease with a prevalence of 4.0 % to 6.0 % in adults worldwide (1) The patient profile is very heterogeneous with different pharmacological and nutritional treatment needs (2) DM involves accelerated atherosclerosis, leading to organ failure due to macro- and micro-angiopathy (3-6).
Disease-related malnutrition (DRM) in DM is a complication that often goes unnoticed. The VIDA study, conducted in hospitalized elderly diabetic patients, showed that the prevalence of malnutrition is higher in diabetic patients than in non-diabetic patients, with malnutrition rates of 21.2 % in diabetic patients and a risk of malnutrition of 39.1 % (7,8). This DRM in diabetic patients leads to an increase in the risk of mortality (9).
Studying the metabolic response after intake is essential for assessing the postprandial situation. It will depend on the glycaemic response, marked mainly by carbohydrate intake, which in turn will depend not only on the type of carbohydrate but also on other nutrients, such as fat, fibre, and protein, in terms of both quantity and quality. This means that the overall composition of the intakehas an impact on the metabolic response of diabetic patients (10).
The ADA 2022 guidelines set out recommendations for a healthy diet following a so-called Mediterranean diet pattern, which is rich in monounsaturated fatty acids, mainly from olive oil, and polyunsaturated fatty acids (omega-3), besides being rich in fibre and low in sodium. Glycaemic load and glycaemic index recommendations should be made on an individual basis according to the patient’s clinical situation (2). In patients at high cardiovascular risk, extra virgin olive oil, in the context of a Mediterranean diet, has shown multiple benefits in the development and progression of diabetes (11,12).
Regarding artificial nutritional support, multiple studies have been conducted to assess the impact of enteral nutrition formulas on the metabolic and glycaemic response in diabetic patients (13-19). Nevertheless, there is currently controversy in clinical practice guidelines about the benefits of formulations specifically designed for diabetic patients (20). The meta-analysis by Sanz París et al (13). investigates the benefits for metabolic control and glycaemic control of formulas with a high intake of monounsaturated fatty acids [>20 % of the total energy (TE)]. It is necessary to develop studies focused on diabetic patients with disease-related malnutrition with an adequate phenotypic and aetiological diagnosis (21).
Continuous interstitial glucose monitoring allows us to assess an individual’s glycaemic response in a more comprehensive way. This technology, which is becoming increasingly widespread in our environment, allows us not only to monitor glucose continuously but also to determine periods in and out of range and to detect silent hypoglycaemia (22). This continuous monitoring has been shown to reduce complications related to the timing, frequency, and intensity of hypoglycaemic episodes (23). Regarding the monitoring of the glycaemic response of malnourished diabetic patients after the intake of nutritional support, these tools may be advantageous for assessing the impact of diet and supplementation on an ongoing basis.
In patients with diabetes and obesity, a study has been published with diabetes-specific dietary substitutes assessing the interstitial glycaemic response using FreeStyle-Libre® technology. In this study, the researchers describe the benefits of a diabetes-specific formula (DSF) as a replacement for breakfast and show that when used as an afternoon snack, it improves both glycaemic control and behavioural factors related to the dietary management of diabetes (24).
Currently, there is no study that has assessed the glycaemic impact of a specific formulation of a diabetes-specific oral nutritional supplement using continuous interstitial glucose monitoring.
The aim of the study was to compare the glycaemic and insulinaemic response of patients with type 2 diabetes at risk of malnutrition after oral feed between a diabetes-specific formula (DSF) and a standard one (STF).
METHODS
From March 2019 to April 2022, we conducted a randomized, double-blind, crossover, multicentre, controlled clinical trial. ClinicalTrials.gov Identifier: NCT05423938.
PATIENTS
Adult patients with a diagnosis of DM2 (confirmed by the use of oral hypoglycaemic agents for at least two months) at risk of malnutrition or malnourished (diagnosed with subjective global assessment [SGA]) were recruited. Patients with DM1 or DM2 on insulin therapy and patients with DM secondary to steroids were excluded. Also excluded were patients on treatment with alpha-glucosidase inhibitors, as well as those with a BMI > 35 kg/m2, malignant neoplasia, advanced chronic kidney disease (glomerular filtration rate < 30 ml/min), severe hepatopathy and gastroparesis, or a chronic infectious disease (active tuberculosis, hepatitis B or C, HIV). Pregnant or lactating women and patients with an allergy or intolerance to any of the ingredients of the formulas could also not participate.
SAMPLE SIZE CALCULATION
Regarding the sample size calculation, the results of a study by De Luis et al. (2013) were used (19) in which a mean difference in peak plasma glucose concentration of 20 % and a standard deviation of 22.97 mg/dL were obtained. At a confidence level of 80 %, 13 patients per experimental arm would be needed. Allowing for a loss to follow-up of 10 %, 14 patients per group are then considered, making a total of 28 patients.
METHODOLOGY
At the initial visit, randomization, SGA, and anthropometric assessment (weight and height) were carried out, besides measuring the body composition with bioimpedance. A continuous interstitial glucose monitor (FreeStyle-Libre®Pro, Abbott®) was placed, and a unique unit of the first oral nutritional supplement (ONS) of 200 ml, whose identity was unknown to both the patient and the principal investigator, was taken. Patients came to the test fasting (between 8 and 12 hours), having ingested an average of 150 g of carbohydrates per day during the three previous days, without consuming alcohol or having practiced intense physical exercise during the previous 24 hours. Blood samples were drawn for glycaemia and insulinaemia determination at 0 minutes, 30 min, 60 min, 90 min, 120 min, and 180 min (± 5 min). On day 8, the intermediate visit was held, and the same procedure as in the initial visit was repeated, but the ONS taken by the patient was the second formula to be studied. On day 15, the interstitial blood glucose sensor was removed.
FORMULAS TO BE STUDIED
The description of each formula is detailed in table I:
– DSF (Bi1 DIACARE® hp/hc, Adventia Pharma): polymeric, hypercaloric, and hyperproteic, with fibre.
– STF (Bi1 CONTROL®, Adventia Pharma): polymeric, hypercaloric, and hyperproteic, without fibre.
Table I. Macronutrient composition of study formulas per 100 ml.
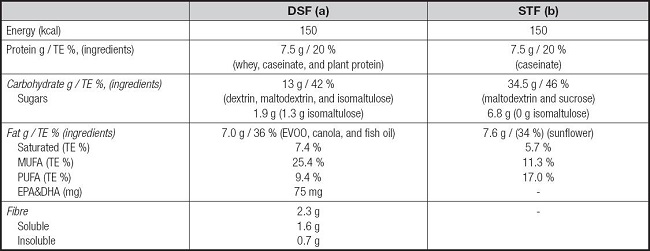
DSF: diabetes-specific formula; EVOO: extra virgin olive oil; TE %: percentage of total energy; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; SFT: standard formula. A: Bi1 diacare® hp-hc, Adventia Pharma S. L., Spain; B: Bi1 control hp-hc®, Adventia Pharma S. L., Spain.
Each oral nutritional supplement (ONS) was presented in a 200 ml blank Tetra Pak® package in exactly the same way for both types of ONS. Each package was labelled with a numerical code as the only differentiation between the two products for the patient to receive either the experimental supplement (DSF) or the control (STF).
LABORATORY TECHNIQUES
The laboratory techniques used for biochemical determinations were: hexokinase for glucosa; chemiluminescence for insulin; bromocresol green for albumin; nephelometry for prealbumin; Latex Enhanced Immunoturbidimetric Assay for Wide-Range for CRP; enzymatic CHOD-PAP method for cholesterol.
RANDOMIZATION
The randomization procedure was performed by the person responsible for the statistical analysis of the study, using a numerical table. Each patient received a participant number so that he or she could start the study with one product or the other (experimental [DSF] or control [STF]) in a crossover manner.
ETHICAL ISSUES
The study was conducted in accordance with the Declaration of Helsinki. The study protocol, patient information sheet, and informed consent were approved by the Málaga Provincial Research Ethics Committee, with the code JGA-DIACARE-2018-01. All patients were informed of the conditions of participation in the study and agreed to participate after signing the informed consent.
STATISTICAL ANALYSIS
For comparison of the glycaemic variability between the two groups, the following parameters were determined: the mean blood glucose, the standard deviation of the mean blood glucose, the area under the concentration curve, the time from nutrition administration to the last measurable glucose concentration (AUC0-t), the maximum glucose concentration (Cmax), and the time to reach the maximum glucose concentration (Tmax). The same determinations were performed with insulin, except for the maximum concentration and the time to reach it. The AUC0-t was calculated by the trapezoidal method (17).
For the analysis, the Student’s t-test was used for continuous variables and the chi-square test for categorical variables. Non-parametric variables were analyzed with Wilcoxon test. For continuous variables, differences between groups are summarized by the 95 % confidence interval (CI 95 %) of the mean difference and the standard deviation (SD), or median and intercuartile range (SPSS Inc. 22.0, Chicago, IL, USA) was used to analyce the data.
RESULTS
DEMOGRAPHIC AND DEVELOPMENTAL PARAMETERS OF TYPE 2 DM
Twenty-nine patients were included in the study (Fig. 1), 51 % female. The mean age was 68.84 (11.37) years. The Charlson complexity index was 4.14 (1.57). The participants had a time course of type 2 diabetes mellitus of 8.66 years (6.89) and an HbA1c of 6.29 % (1.12); the treatment received was mostly metformin (93.1 %), combined, in some cases, with GLP1 analogues and IDPP-IV.
NUTRITIONAL STATUS
Of all the patients, 86.2 % were at nutritional risk (SGA = B), and 13.8 % were severely malnourished (SGA = C). The percentage of weight lost was 8.66 % (5.76), and the patients had a BMI of 24.88 kg/m2 (4.52), a fat mass (FM) of 24.52 % (8.53), and a fat free mass (FFM) of 75.48 % (9.33). Regarding blood tests, an albumin level of 4.08 mg/dl (0.54), a pre-albumin level of 27.22 mg/dl (8.66), a CRP of 3.52 (1.76), and a cholesterol level of 162.92 mg/dl (49.18) were determined.
ANALYSIS OF PLASMA GLUCOSE
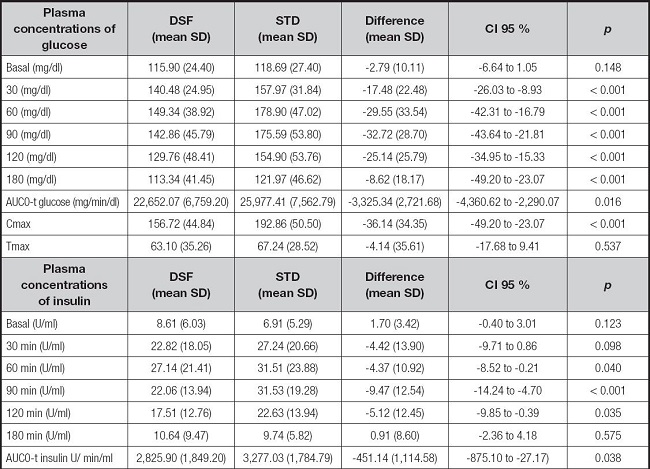
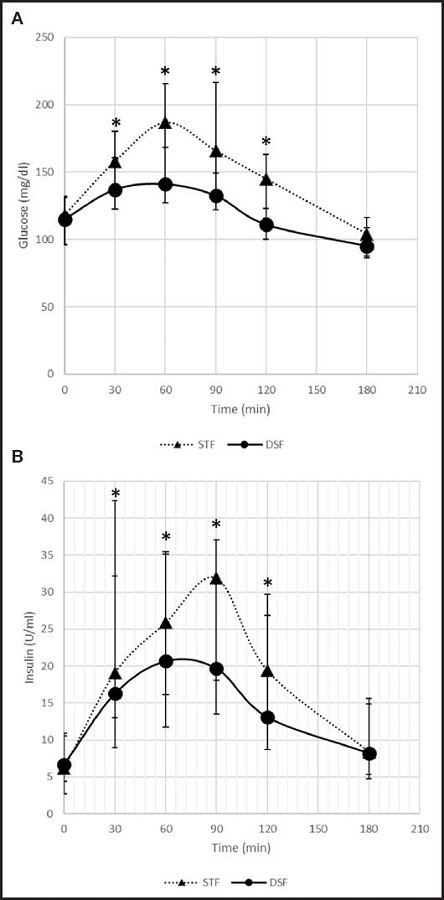
Analysis of the kinetic parameters of glucose concentration depicted in table II indicates that patients treated with DSF had a significantly lower mean of AUC0-t, Cmax and plasma glucose at each measured time, than with STF (Fig. 2). In addition, no significant differences were observed in period, sequence effects, or nutritional status.
ANALYSIS OF PLASMA INSULIN
The analysis of the kinetic parameters of plasma insulin concentration is presented in table II. It is observed that patients treated with DSF had a significantly lower mean AUC0-t and plasma insulin at each measured time, than patients who received STF. In addition, no significant differences were observed in period, sequence effects, or nutritional status.
DETERMINATION OF CONTINUOUS INTERSTITIAL GLUCOSE MONITORING
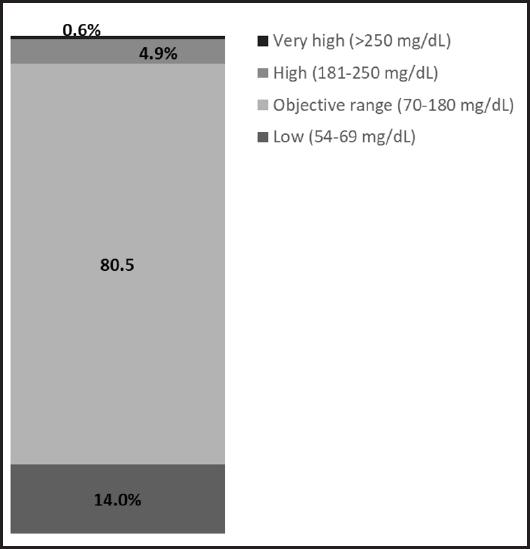
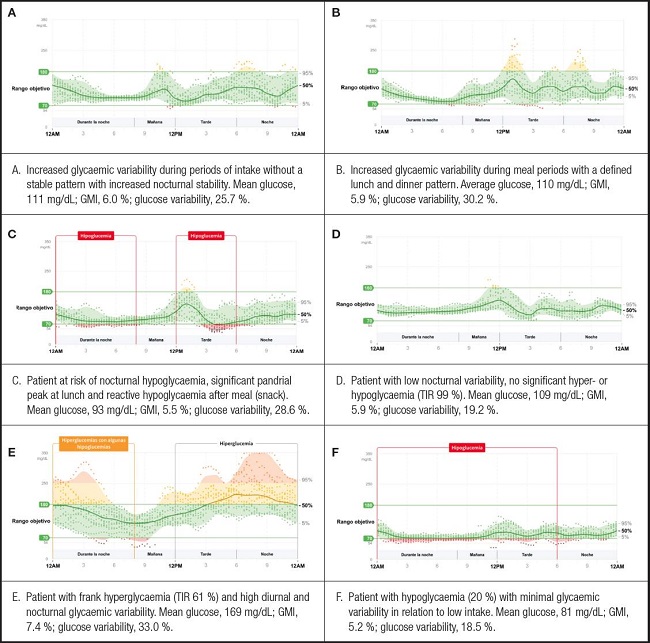
The results of the continuous interstitial glucose monitoring of the patients evaluated showed a mean blood glucose of 104.06 mg/dl (28.59), with an interquartile range of 35.13 mg/dl. The glucose management indicator (GMI) was 5.8 %, and the glucose variability was 27.1 %, defined as the percent target coefficient of variation (% CV) ≤ 36 %. Time in the range, high, and low values are summarized in figure 3. The different interstitial glucose profiles are depicted in figure 4.
DISCUSSION
The DSF with EVOO, designed to meet the specific needs of diabetic patients, has been shown to achieve a better glycaemic and insulinemic response in diabetic patients at risk of malnutrition.
The impact of DSF on glycaemia, compared to STF, was a lower AUC0-t value. These differences in glycaemia may be explained by the difference in carbohydrate intake, not only in quantity but also in the carbohydrate mix. In DSF, the use of isomaltulose and the mixture of dextrin and maltodextrin, with a low DE index, has been shown to be effective in the matrix of the enteral formula, improving the glycaemic response compared to the standard formula. In this case, the presence of fibre in DSF has also conditioned this better response, as has been shown in studies carried out in the same field (25). These data agree with other previously published studies evaluating the impact on glycaemia of enteral diets specifically formulated for diabetic patients. In a study by De Luis et al. (19), carried out in patients with type 2 diabetes mellitus without malnutrition, a difference in AUC was observed in favour of DSF compared to STF (mean difference: -4,753.26 mg min/dL [95 % CI: -7,256.69 to -2,249.82]; p = 0.001). This greater difference than that detected in our study can be explained by the characteristics of the standard formula used in this study, as it had a lower percentage of fat and carbohydrates than the formula used by us. In a study by Lansink et al. (2016), the researchers also observed statistically significant differences in blood glucose AUC0-t, with the measurement in the DSF group being lower than that in the STF group (167.6 mmol/L min [121.1] vs. 515.6 mmol/L min [181.1]; p < 0.001). A study by Alish et al. (2010) also shows that DSF improves the glycaemic response in diabetic patients by reducing AUC0-t compared to STF (1,690.7 mg min/dL [431.5] vs. 7,460.3 mg min/dL [1,074.9]; p < 0.001) (15).
The impact of DSF with EVOO on insulinaemia, compared to STF, resulted in a lower AUC0-t value. These data agree with other previously published studies evaluating the impact on insulinaemia of enteral diets specifically formulated for diabetic patients. In a study by De Luis et al. (2013), a difference in AUC0-t in favour of DSF compared to STF was observed (mean difference: -930.27 µU/mL/min [CI 95 %: -1,696.34 to -164.2]; p = 0.039) (19). In a study by Lansink et al. (2016), the researchers also observed statistically significant differences in the AUC0-t of insulinaemia, with the measurement being lower in the DSF group than in the STF group (4,446.7 pmol/L min [3,021.6] vs. 7,336.6 pmol/L min [5,134.4]; p < 0.001). A study by Alish et al. (2010) also shows that DSF improves the insulinaemic response in diabetic patients by reducing the insulinaemic response (AUC0-t) of diabetic patients compared to STF (4,723.1 µU/mL/min [1,001.7] vs. 9,050.8 µU/mL/min [1,869.1]; p < 0.001).
Regarding the impact of a monounsaturated fat-rich DSF on glycaemic control and nutritional status in the medium to long term, the study by De Luis et al. 2008 evaluated the impact of two supplementation doses (2 or 3 packs/day). The authors concluded that DSF is effective in improving HbA1c and nutritional status, especially when the pattern is with three packs per day (26). It would be interesting to propose studies with EVOO-rich DSF to assess its metabolic impact and nutritional status in the medium to long term in chronic patients with nutritional support.
The studied DSF had a lower insulinaemic response, which is beneficial per se. The data collected on insulinaemia highlight the differences in the maximum peak insulinaemia at minute 60 in the DSF group, respect to STF that it was at minute 90, as well as in the AUC0-t. These elevated insulin levels in the standard diet are associated with increased cardiovascular risk, not just hyperglycaemia. We know that this insulinaemic response is conditioned by dietary fat intake (type and amount), fibre intake, protein quality, and carbohydrate intake (type and amount), as indicated in the ADA 2022 guidelines (2). Considering the type of DSF evaluated, the contribution of extra virgin olive oil, both in terms of its high content of MUFA (27) and in terms of its bioactive phenolic compounds (28), the high content in whey protein, which promotes insulin sensitivity (29), the fibre, and the specific carbohydrate mix, may account for this better insulinaemic response compared to STF.
The patients presented in this study were old, had a long history of DM2, and had a high comorbidity index. The patients evaluated had good metabolic control, with an HbA1c value within the optimal control parameters, but this situation may be caused by malnutrition (30).
The assessment of the nutritional status with the SGA has been the gold standard for the diagnosis of malnutrition in recent years until the appearance of the GLIM criteria (31). In our study, among the variables assessed by the SGA, it was weight loss, decreased lean mass, and decreased fat mass, as well as decreased dietary intake, that determined the diagnosis of malnutrition, and not BMI. In this regard, we observed that malnourished diabetic patients have BMI values within the ranges of normal weight, overweight, and type 1 obesity, already identified in previous studies, such as the VIDA study (7). This implies that a nutritional diagnosis using BMI alone in type 2 diabetic patients may underestimate the prevalence of malnutrition (7).
This is the first study to analyse the interstitial glycaemic response through continuous monitoring using the FreeStyle-Libre®Pro system to assess the effect of ONS in malnourished diabetic patients. Continuous monitoring over 14 days not only measures the impact on blood glucose of the supplement under study and control, but also includes the patient’s dietary intake. In this study, only aggregated data are shown, but it was observed that there was a 14 % of the time below 70 mg/dl. It could be a wake-up call that patients with malnutrition and type 2 diabetes may present episodes of hypoglycaemia, and need to be monitored properly. One of the advantages of using these devices in the monitoring of diabetic patients on enteral nutrition is to ensure adequate glycaemic control and the detection and prevention of hypoglycaemic episodes (23).
One of the strengths of the study is that it is the first study to evaluate the glycaemic and insulinaemic response of DSF with EVOO. EVOO is a food that has demonstrated multiple benefits in patients at high cardiovascular risk and, specifically, in diabetic patients (25). Also, for the first time, continuous monitoring of interstitial glucose in malnourished diabetic patients was included, which, although it was not compared with plasma measurements (because it was not the objective of the study), was used to evaluate glycaemic control, time in range, and episodes of hypoglycaemia.
Regarding limitations, the software of the FreeStyle-Libre®Pro device did not allow us to obtain specific interstitial glycaemia in the same period as plasma measurements. The sample size of 29 patients was larger than that in other studies and was compensated for by the fact that it was designed as a crossover clinical trial. It should be noted that the prolonged time involved in patient recruitment was the lack of awareness of the detection of malnutrition in the outpatient care of DM2 patients by healthcare professionals.
CONCLUSIONS
The diabetes-specific formula for diabetic patients, formulated with extra virgin olive oil, a specific mixture of carbohydrates, fibre, and rich in whey protein, not only benefits glycaemic control but also improves the insulinaemic response in patients with type 2 diabetes mellitus at risk of malnutrition.