INTRODUCTION
Fungal infections such as candidiasis are one of the major causes of morbidity and mortality among pediatric hospital infections, with a mortality rate that can reach 50 % (1,2). Premature birth is a common occurrence both in developed and developing countries, but survival is lower in countries with low and median income. Prematurity is associated with a higher risk of sepsis, cardiovascular abnormalities, respiratory ailments, as well as gastrointestinal and neurological complications (3).
In the nutritional care of the premature newborn, parenteral nutrition is often used in an attempt to simulate intrauterine growth. Protein nutrition is especially important, and is often achieved with protein supplements in addition to breast milk (4,5). Essential amino acids should be included to protein metabolism, and support growth and development (6).
Nutrition of the premature newborn is vital to the goals of achieving normal weight, tolerating the enteral delivery route, and minimizing hospitalization time (3). Glucose is normally administered between 6 and 8 mg/kg/min as soon as possible after birth and adjusted based on frequent measurements of plasma glucose to maintain such levels above 45 mg/dl, but below 120 mg/dl, and avoid periods of hypo- and hyperglycemia. Similarly, lipids should be provided at a minimum of 0.5 g/kg/day; they provide energy and prevent deficiency of essential fatty acids (6-9), especially with the use of fish oil in the lipid formulation (10-12).
Among the macronutrients included in parenteral nutrition, the lipid solutions are most at risk for microbial contamination, especially fungal contamination by Candida albicans. To help prevent such contamination, good hygiene practices should be followed and parenteral administration should be limited to 24 hour periods (13,14).
Contamination with Candida albicans during parenteral nutrition requires the use of antifungal medication which can prolong hospitalization and increase mortality risk, especially among newborns (15,16).
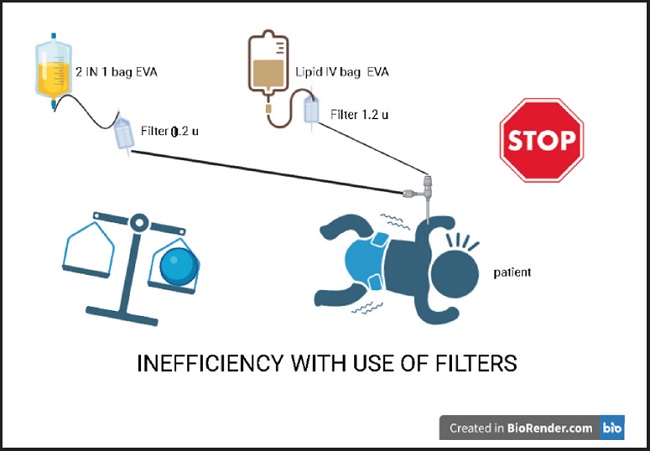
The intravenous filter is a device recommended in parenteral mixtures. Typically, either the 1.2 µm filter is used for 3 in- 1 bags, those formulations that contain the 3 macronutrients (carbohydrates, amino acids and lipids) and micronutrients. In contrast, the 0.2 µm filter is recommended for the 2 in-1 bags that contains only 2 macronutrients (carbohydrate and amino acid) with a separate use of intravenous lipids (17).
Despite these recommendations, in some health facilities, filters are not used, and when they are used, inappropriate practices are common, for example, the inappropriate use of 0.2 µm filter for the 2-in-1 bags, and connecting the intravenous lipids in the ethyl vinyl acetate bag EVA directly to the 1.2 µm filter, and then to the peripherally inserted central catheter installed in the premature newborn (Fig. 1).
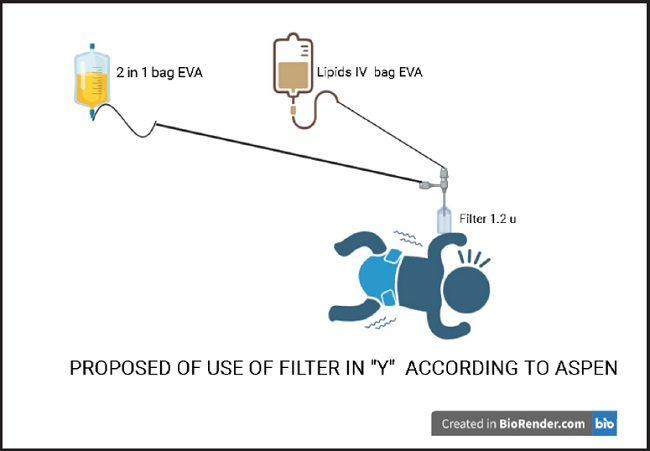
The inadequate use of the 1.2 µm filter can lead to economic losses within a health system that is already severely financially stressed, and does not guarantee greater safety for the newborn. The extra costs must be assumed by a family member or by the state. Such extra costs could be avoided by following international recommendations to administer 2 in 1 parenteral nutrition, the in-line “Y” (17-21) structure as shown in figure 2.
In the context of such considerations, the main objective of this study is to demonstrate that the common use of two filters (Fig. 1) represents a waste of resources and funds and does not contribute to safety in terms of preventing Candida albicans infections in newborns. In this study, the devices containing intravenous lipids administered in premature neonates were tested for the presence of Candida albicans and the relationship with the correct use of 1.2 µm filter was analyzed.
MATERIALS AND METHODS
SELECTION CRITERIA
In a Neonatology facility of the Ministry of Health, information was obtained about premature patients who required parenteral nutrition and whose healthcare service was outsourced for three months over a winter season.
INCLUSION CRITERIA
Residues of lipid solutions from EVA bags used for parenteral nutrition of premature patients hospitalized in the neonatal service, patients aged 3 to 40 days and weighing less than 2000 g.
EXCLUSION CRITERIA
Residues of lipid solutions contained in EVA bags of children who did not receive parenteral nutrition with lipids; EVA bags with too little residual sample; EVA bags used on weekends where there were no qualified personnel for sampling and whose bags were unintentionally discarded or not collected on the same day.
Samples analyzed
− First phase: samples were taken from 34 bags of ethylvinyl acetate (EVA) selected before filtering the lipids and after filtering. Samples were then seeded in Merck Sabouraud Agar, and readings performed at 48 h and 72 h. As a positive control, a strain identified as Candida albicans obtained from the National Research Laboratory I was planted in plates of Merck Sabouraud Agar with readings also at 48 h and 72 h.
− Second phase: bags that resulted in a negative culture for Candida from the first phase were collected and exposed to contamination with the strain of Candida albicans sp. The contaminated sample was seeded in Merck Sabouraud Agar plates until they gave a positive Reading. After passing through the 1.2 µm filter, the filtered sample was again seeded in the same culture medium and read at 48 h and 72 h.
RESULTS AND DISCUSSION
To avoid the risk of postpartum malnutrition, parenteral nutrition must provide the necessary nutrients — carbohydrates, protein, lipids, and micronutrients — to support growth and development of the newborn. Lipids not only provide calories but also encompass the essential fatty acids which support body growth, development of the immune system, protect against inflammation, and have a range of other benefits (20).
Table I shows the data of premature patients weighing less than 1800 g who received parenteral nutrition. Due to their physiological condition, these patients are more likely to have some complications and risks of infection (3). The 10 premature patients, before receiving parenteral nutrition, they did not present infections that required the use of antifungals. It is possible that contamination of patients with Candida may come from handling of the catheter and the patient. This contamination must be avoidable, taking the same precautions of Good Practices in the handling of all the material to be used and in the patient´s environment, as well as in the administration and preparation of parenteral nutrition to preserve the non-infections state (13,14).
Table I. Details of the premature newborn patients that received parenteral nutrition during this study.
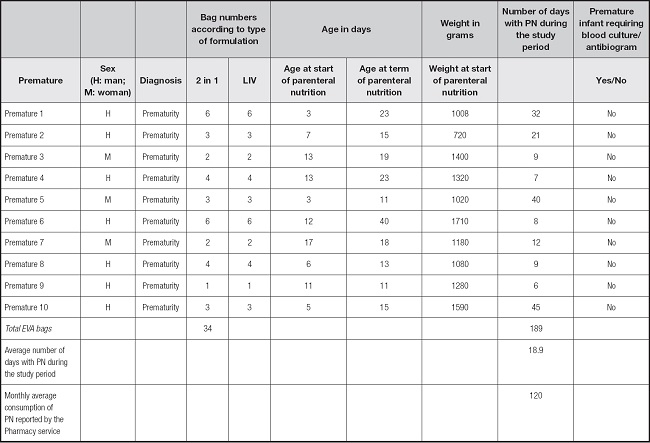
LIV: intravenous lipid; EVA: ethyl vinyl acetate; PN: parenteral Nutrition; Number of days with PN: number of bags PN that are installed for 24 hours.
Table II shows that the intravenous lipid samples obtained directly from the EVA bag, (ML1) were all negative, even without the use of the 1.2 µm filter. These data show that filter use would not be necessary in this step, unless the manufacturer does not comply with the quality control within its manufacturing processes, to avoid microbial contamination and formation of large macromolecular aggregates greater than 1.2 µm (13,14,21). As expected, after passing the bag solution through the 1.2 µm filter (ML2), all samples were again negative for Candida albicans.
Table II. Presence of Candida albicans in lipid devices with and without filtration.
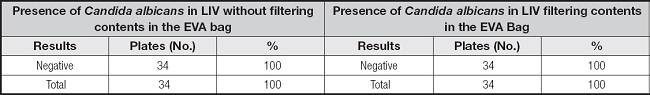
EVA: ethyl vinyl acetate; LIV: intravenous lipid.
There can be differences in recommendations made by professional associations, manufacturers of products for parenteral nutrition, and by companies that outsource the mixtures, on the use of the 1.2 µm filter for intravenous lipid solutions when administering 2 in 1 bags and lipids in EVA bags; and this has generated some controversy (22-24).
Recommendations typically mention using a 0.2 µm filter for 2-in-1 parenteral nutrition that contains only two macronutrients such as carbohydrates and amino acids in addition to micronutrients. The function of such filters is to prevent bacterial contamination and retain particles larger than this diameter. Likewise, the 1.2 µm filter for 3 in 1 mixtures that also includes lipids or for 2 in 1 mixtures with lipids contained in another bag is typically recommended to be administered using an in-line “Y” setup (3,21-25).
The data in table III support the benefit of the filter in terms of preventing microbial infection: induced contamination with Candida albicans in the EVA bag containing intravenous lipids was retained in the 1.2 µm filter, and all tests for Candida downstream of the filter were negative (22,24).
Table III. Presence of Candida albicans with and without filtration in lipid devices that were intentionally contaminated.
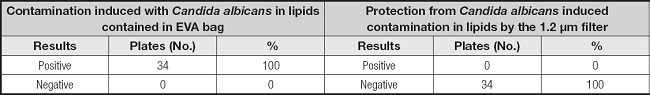
EVA: ethyl vinyl acetate.
Operating procedures during the development of parenteral nutrition must meet the minimum technical requirements to ensure the safe use of this therapeutic intervention (26,27). Some reviews mention parenteral nutrition as a predisposing factor for Candidemia (28); however, prospective studies that ensure the presence or absence of microorganisms need to be considered (29). According to Austin et al. (9), lipids in parenteral nutrition do not increase Candida growth since they are administered over a 24-hour period and then device and bag are changed. On the other hand, Gavin et al. (30), showed that by inducing Candida contamination in lipid-containing parenteral nutrition bags, microbial growth was found after moving the bag to a central Access position; thus, they recommended daily device switching.
Table IV shows the comparative costs of using one or two filters for the administration of parenteral nutrition. The total cost has been calculated taking as a reference the average monthly consumption of parenteral nutrition bags reported by the Pharmacy service, either quarterly or annually when using the 1.2 µm filter for the lipid bag in addition to the 2 in 1 bag with the 0.2 µm filter, finding that it would spend twice as much when using 2 filters. It is more economical and efficient to use 1.2 µm in-line filter when using binary parenteral nutrition with separate intravenous lipids; this efficiency includes optimizing human resources because the placement of 2 filters requires additional time from the nursing staff and additional materials (31). Incorrect practices with the use of 1.2 µm filters imply an expense to the country's health system that is three times greater than tan with proper use for the same procedure.
Table IV. Costs using on line filter “Y” and not “Y” during the study time and their quarterly and annual projection.
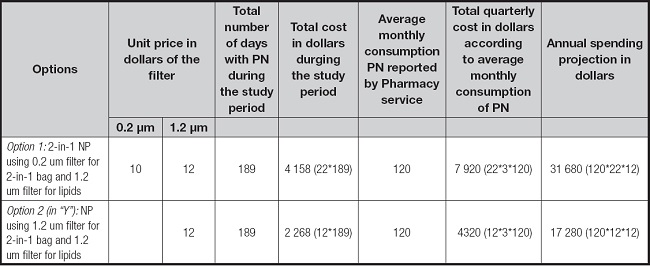
EVA: ethyl vinyl acetate; PN: parenteral nutrition.
In terms of future research, it is necessary to consider the factors that can favor growth of Candida. When placing the lipids in the bag a single manipulation is carried out and the transfer is made within a closed circuit. In this context, our small-scale study supports the recommendation of the American Society of Nutrition and Parenteral (ASPEN) on the in-line use of the 1.2 µm filter when the mixtures are binary and not for intravenous lipids alone. Future research will also determine the relative efficiencies of the different parenteral delivery setups in terms of large macromolecular aggregates.