INTRODUCCIÓN
Nutritional status and quality of life (QoL) in cancer patient are frequently affected by the host response to tumor growth, as well as by antineoplastic treatment. These patients often experiment weight loss and poor appetite, malnutrition and, consequently, cancer-related cachexia (1). The negative energy balance, systemic inflammatory responses triggered by tumor activity have been related to an increase in resting energy expenditure (REE), that is widely considered as one of the major determinants in the development of malnutrition in cancer patients (2).
The European Society for Clinical Nutrition and Metabolism (ESPEN) recommends indirect calorimetry (IC) to determine REE in the clinical settings and also to estimate total energy expenditure (TEE), which is the result of REE+ physical activity (PA). If it is not available, international guidelines recommend calculating calories in cancer patients according to 25-30 kcal/kg of body weight per day because of the high prevalence of elevated metabolism in cancer patients. However, there is a low amount of scientific evidence that evaluates the applicability of these indications to the ambulatory attention of patients with cancer, specifically women (3).
In normal population, metabolism decreases after 60 years (4). In cancer, some studies report variations in energy expenditure. While some authors showed that less than 70 % of the patients could present an elevated resting energy expenditure or hypermetabolism compared with predictive equations (REE > 110 % of predictive equations), reports are indicating that 30 % of patients could experience some decrease on REE or hypometabolism, which is associated with advanced disease (REE < 90 % of predictive equations) (4-6).
Several clinical alterations are related to REE changes in cancer population (7,8). Factors related with an increased REE are tumor type (lung, head and neck, pancreas, liver, gastrointestinal or prostate cancer), clinical stage, extensive abdominal injuries, central nervous system flux, metastasis and infections. At the same time, hemodynamic instability, bioenergetic cell failure, low percentage of lean mass or malnutrition, sarcopenia, and hypothyroidism are related to a decreased REE in this population (7,9-11).
Gynecological and breast cancer are common malignancies in women but research on their metabolic implications is scarce. In women with cancer, an increase in weight during antineoplastic treatment is frequently observed, which make this population more variable according to metabolism (11,12).
Few studies investigate the specific changes of REE during cancer treatment in women that suggest the possible adequation to an antineoplastic treatment. Some follow-up studies in cancer patients during antineoplastic therapy showed different types of responses during treatment in both sexes. Chemotherapy (CT) had mixed results, but radiotherapy (RT) showed mainly a decrease in REE of patients (13). However, due to the lack of information comparing treatments with REE, the variability of patient characteristics, and the small sample size of the studies, this question has not been answered at all (14-16).
This study aimed to determine the changes of REE and QoL after the first cycle of antineoplastic treatment in female patients with gynecological cancer and to compare the agreement with what it is stated in the ESPEN guidelines.
METHODS
PATIENTS CHARACTERISTICS
This prospective, observational cohort study enrolled female patients with a gynecological cancer diagnosis from the Oncologic Ward of a public referral tertiary-level hospital. The study was registered and approved by the ethics and research committee of the hospital with the registration number DI/13/111/04/005. All patients signed an informed consent to participate in the study. Inclusion criteria considered women between 18 and 60 years, programmed to receive chemotherapy, radiotherapy, or a combination of both for the first-time during the period 2016-2019. Patients who reported surgery for tumor extirpation (not biopsy) three months before evaluation and those with poor respiratory status as well as those with were not included.
Patients were divided into three groups according to the antineoplastic treatment needed and prescribed by a clinical oncologist: CT, RT, and concomitant chemoradiation therapy (CRT). Evaluation after treatment was developed four weeks after the stated treatment.
NUTRITIONAL ASSESSMENT
All clinical data were collected from the medical records. A dietitian conducted nutritional assessment before (T0) and after treatment (T1). This measurement included: weight (kg) and height (m) using a SECA 762 scale and a SECA 213 stadiometer measured according to a standard protocol described, respectively (17). Body mass index (BMI) was calculated at baseline and after treatment (18).
INDIRECT CALORIMETRY
Resting energy expenditure (REE) was evaluated in all patients before and after the first cycle of antineoplastic treatment. Indirect calorimetry (IC) equipment used was an REE VUE, KORR® calorimeter (Korr Medical Technologies, Salt Lake City, UT), a viable alternative for clinical medical evaluation (19,20).
Evaluation of REE was carried out under standard resting conditions. All patients followed an 8- to 10-hour overnight fasting prior to the calorimetry assessment. The calorimeter device was automatically calibrated before each measurement while the patient was sitting on a chair with a 15-20 minutes period of resting before the assessment to acclimate all participants to the instrument conditions. The standardized measurement lasted ten minutes and required the patients to breathe through the mouth via a mouthpiece connected to a closed loop of the calorimetric system. A nose clip was used to avoid air movement through the nose. Data obtained from calorimeter analysis included oxygen volume consumption in litters per minute (VO2/min) and per kilograms (VO2/min/kg), fraction of inspired oxygen (FiO2) and REE (kcal/day and kcal/kg).
QUALITY OF LIFE
The QLQ-30 questionnaire developed by the European Organization for Research and Treatment of Cancer (EORTC) was used to estimate QoL according to the QLQ-30 instruction manual available for free download in https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf. The QoL-30 report included items like global QoL, physical function, role function, emotion, cognitive function, social, fatigue, nausea and vomiting, pain, dyspnea, insomnia, constipation, diarrhea, loss of appetite, and financial difficulties. Each item gave a score between 0 to 100, where the higher the score, the higher the functioning level.
RESTING ENERGY EXPENDITURE ACCURACY ACCORDING TO GUIDELINES
According to the ESPEN guidelines requirements, energy values were estimated for 25 kcal/kg and 30 kcal/kg (3), and compared with the TEE estimated (REE+ physical activity) according to the classification of Boothby W et al.: hypometabolic or underpredicted (REE < 90 % of predicted), normal metabolism or accurate (90-110 % of predicted), and hypermetabolic or overestimated (> 110 % of predicted) following the ESPEN range recommendation (21).
STATISTICAL ANALYSIS
Results have been expressed as percentages, averages, and standard deviation (± DS) according to the variable type. The statistical analysis was performed using the SPSS program 22th version (IBM Corp. 2013, Armonk, New York, United States) and R software version 3.4.1. General analysis was developed using a Student's t test for paired samples to compare numerical variables before and after each type of antineoplastic treatment. Determination of differences in the indirect calorimetry parameters between groups prior to and after treatment was performed with ANOVA test for normal distribution variables and Kruskal-Wallis test for non-normal variables. Student's t test for paired samples was considered for the independent analysis of each group and Wilcoxon test was used for those variables with non-normal distribution. Bland-Altman analysis was used to estimate the accuracy of the energy determined through REE compared to ESPEN guidelines plus the confidence intervals (95 %). The analysis considered p-values < 0.5. García-García JA et al. recommendation of including between 30 and 50 participants, who must have the attributes that are to be measured in the target population, to calculate sample size was considered (22).
RESULTS
This study included 54 women with gynecological cancer. Figure 1 describes the flowchart of patients recruited in the study. Proportion of patients according to the antineoplastic treatment group was: 31.5 % (n = 17) for the QT group, 37 % (n = 20) for the QRT group, and 31.5 % (n = 17) for the RT group. The mean age of the total population was 48.2 ± 9.1 years. Global QoL of the total population was 65.8 ± 20.4 % from a range of 0-100 %. Clinical characteristics shown in table I indicate that cancer type, clinical stage, and BMI classification were homogenous among groups.
Table I. Clinical characteristics of cancer patients before antineoplastic treatment.

n = 54. REE: resting energy expenditure.
*p-values using ANOVA test.
ap-values < 0.05 using Kruskal-Wallis test.
ANTHROPOMETRIC ASSESSMENT
Means of weight in total population of women in T0 and T1 was 61.4 ± 10.7 kg and 59.4 ± 9.6 kg, respectively. After treatment, there was a significant mean of weight change, with a weight loss of -2 ± 45 kg (p = 0.005). General population at T0 presented a mean of height of 1.55 ± 0.06 m without variation in T1 (p > 0.05). Statistical differences were showed in the means of weight and BMI after treatment (p = 0.004), with mean values of 25.7 ± 4.9 at T0 and 24.8 ± 4.3 kg/m2 at T1. Body weight changes were observed in 88.9 % of the total population: 55.6 % (n = 30) of patients experimented with weight loss, 33.4 % (n = 18) gained weight, and 11.1 % (n = 6) conserved the initial body weight.
According of groups of intervention, at T0, the CT group presented the highest means of weight and BMI compared with RT and CRT (Table II).
Table II. Anthropometry and indirect calorimetry assessment of female patients with gynecological cancer before and after antineoplastic treatment.

BMI: body mass index; REE: resting energy expenditure; VO2: oxygen volume; FeO2: exhaled oxygen fraction.
*p-values using ANOVA test.
†p-values < 0.05 using Kruskal-Wallis test.
INDIRECT CALORIMETRY ASSESSMENT
In total population of women, means of REE and REE adjusted per kg at T0 were 1,328.5 ± 369.6 kcal and 22.1 ± 6.6 kcal/kg. Table I showed that cancer type, clinical stage and BMI did not influence the REE of our population prior to starting antineoplastic treatment. However, table II shows that, among groups of intervention, there was a significant difference in means of REE (p < 0.05) but this was not observed when REE was adjusted by kg of body weight (p > 0.05).
After treatment, means of REE and REE/kg were 1,201.7 ± 351 kcal/d and 20.8 ± 6.9 kcal/kg, respectively. Even though there was a significant reduction of 127 (-10.5 %) kcal comparing T0 versus T1 (p = 0.018) in general population, there were no statistical differences between groups of intervention in means of REE, REE/kg, FeO2%, and VO2 (ml/min) at T1 (p > 0.05). VO2 adjusted per minute and kg showed statistical differences between groups; CT had the lower values. Table II shows the IC assessment at T0 and T1 of each antineoplastic treatment.
QoL ASSESSMENT
QoL mean after treatment was 66.3 %. QoL did not reported statistical changes after treatment in general population (p = 0.884) nor according to groups (p = 205). Figure 2 showed results of emotional and functional scale before and after treatment in general population.

Figure 2. Quality of life at T0 and T1 of antineoplastic treatment in total women population. T0: before treatment; T1: after treatment.
Before treatment, CT had 65.2 ± 20.2 % and CRT had 60.8 ± 20.7 %, showing lower QoL compared with the RT group, which presented 80.3 ± 16.3 % (p = 0.006). However, after treatment groups showed a QoL mean of treatment with homogenous means among groups (p > 0.05): CT 67.8 ± 20.6 %, CRT 59.7 ± 23.6 % and RT 73.3 ± 20.9 %.
ACCURACY OF REE COMPARED WITH RECOMMENDATIONS
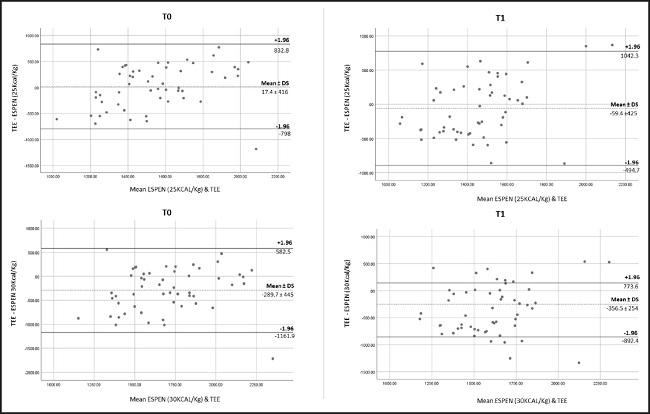
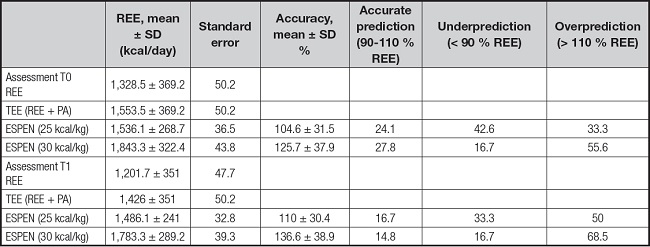
Results of the agreement between ESPEN recommendations of total energy expenditure and the values of TEE obtained by measured REE by IC of women with cancer before and after the first cycle of treatment are shown in table III. Accuracy of energy estimation according to 25 kcal/kg and 30 kcal/kg was present in less than 30 % of patients. The means of accuracy showed to be upper using values of 30 kcal/kg compared with 25 kcal/kg in both times: T0 and T1. Bland-Altman analysis comparing the differences between ESPEN range of recommendations and TEE values presented the mean differences, or bias for T0 and T1 (Fig. 3). The 95 % confidence interval of means for TEE-25 kcal/kg at T0 and T1 were -96.3 to 131.24 kcal and -176 to 56.78 kcal, respectively; and for TEE-30kcal/kg, they were -411.4 to -168.1 kcal and -481.2 to -233.1 kcal at T0 and T1, respectively. Our results showed that energy requirements estimated with 25 kcal/kg had better agreement at both T0 and T1, compared with 30 kcal/kg according to the 95 % confidence intervals in women population with cancer.
Table III. Agreement between ESPEN recommendations and IC of women with cancer before and after the first cycle of treatment.
IC: indirect calorimetry; ESPEN: European Society for Clinical Nutrition and Metabolism; REE: resting energy expenditure; TEE: total energy expenditure; PA: physical activity; T0: baseline; T1: after first cycle of treatment. n = 54.
DISCUSSION
REE is variable in cancer patients and it is not clear how antineoplastic treatment affects it. The principal aim of this study was to determine the changes of REE and QoL before and after antineoplastic treatments in women. Our findings showed that women's REE and QoL decreased after the first cycle of antineoplastic treatments, but this change was not associated with a particular antineoplastic treatment (Table II). Moreover, QoL showed an increase in physical symptoms like fatigue, nausea, pain, dyspnea, constipation, diarrhea, loss of appetite and economic differences, which is related to malnutrition and worse clinical course (23).
Patients in the chemotherapy group experimented the higher values of REE and those in radiotherapy presented a slight increase of REE after the first cycle of radiotherapy sessions.
REE has variable reports; some showed no changes whereas others showed an increase in REE (14-16,24). In female population with cancer, research about metabolic implications during antineoplastic treatments is scarce (25).
Even though no differences were observed at T1 among groups, T0 showed unexpected differences between groups, showing that CT group had a higher REE compared with RT and CRT. This could be explained because our low sample size between groups and this group had the highest BMI and after treatment where the group that lost the major quantity of body weight (3.3 kg). This is supported by a study in population from the Netherlands that assessed 513 general hospital patients and found that BMI was associated with REE, so that REE increased according to the degree of BMI (26).
The most common studies are applied to the general population, and tumors leading to cachexia. A study of Langius JAE et al. (16), conducted in head and neck cancer patients during RT, reported no statistical differences in REE compared to controls at the beginning of the study, but REE decreased continuously with ongoing weight loss in the first week, and recovered at the end of RT sessions. Our study showed a trend towards an increase in REE in the RT group at four weeks of sessions, but REE was not measured weekly to detect the time when the change in REE was observed.
A study conducted by García-Peris P in 18 head and neck cancer patients showed that REE was significantly higher before and after at two weeks of chemotherapy treatment compared with patients at radiotherapy. Despite its low sample size, this study also a found a significant weight loss in its population (13). This could be possible because they did not report a significant reduction in weight, and some other studies relate the amount of fat-free mass and energy expenditure (24). In our study, all study groups patients experienced a significant weight loss (p < 0.05), but this does not affect results after treatment for finding differences among groups at the end but in the 33.4 % of total population there were observed weight gain, as the literature described it is observed in more than the 50 % of women in chemotherapy for the treatment to gynecological or breast cancer (27).
A secondary aim was to compare the TEE calculated by the REE by IC plus the 10 % of PA compared with the 25-30 kcal/kg recommended by the ESPEN society (3). Our findings showed that energy estimations could be overestimated when using 30 kcal/kg. Variation in energy could be possible when using kg for predicting REE because it has been demonstrated that energy expenditure of body weight is influenced by the thermic effect of food, and also by the distribution of adipose and musculoskeletal tissue, leading to inconsistencies when energy requirements are estimated by body weight (28,29). A study conducted by Purcell SA et al. measured REE in patients without antineoplastic treatment and found that REE measured by IC is affected by age and body composition (8). Despite body composition was not measured in our study, 50 % of our total female population presented excess body weight and the other half presented low BMI. These extremes of weight can influence our results regarding the estimation of energy expenditure and TEE (4,24).
Considering that to our knowledge this is the first study comparing TEE with the guidelines recommendations in female population with cancer, our findings are similar to those studies using predictive equations in both sexes, which often conclude do not predict accurately the REE of patients compared to IC (30). The Bland-Altman analysis plot shown in figure 3 describes that the better agreement was using 25 kcal/kg at T0 and T1; however, this can be influenced by the heterogeneity of our population according to the BMI that presented wide values ranges. Also, TEE was estimated with the same PA factor for our female population, leading to variations in the estimation of TEE. The impact of PA on TEE is supported by Moses AGW et al., who showed that PA is a determinant in TEE after eight weeks of follow-up in patients with pancreatic cancer (31).
The limitation of this pilot study was that our IC did not calculate the respiratory coefficient that indicates the type of energy substrate used in the patients neither we assessed individually PA during antineoplastic treatments. Moreover, this study was not planned to evaluate a complete regimen treatment follow-up that includes approximately eight treatment cycles to determine whether the changes occurred throughout the treatment or at a specific point in the treatment. For future research, it is necessary to consider that determining body composition is indispensable to establish if the changes in REE are determined by this factor or PA measured individually at the beginning but also throughout antineoplastic treatments. In addition, is necessary to implement a better understanding of the factor influence REE in female patients with cancer considering age, clinical stage, during antineoplastic treatments in a long-term follow-up.
Estimation of energy requirements in cancer patients, specifically in female population during antineoplastic treatment, is essential in clinical practice but it is still controversial due to the several factors that influence REE. This pilot study conducted in women with cancer during the first cycle of antineoplastic treatments showed that REE decreased after oncology treatments independently of the type of therapy. ESPEN recommendations presented a better mean accuracy using 30 kcal/kg in 28 % of female population, but agreement according to Bland-Altman analysis was better using values of 25/kcal/kg. More research in a bigger sample size of women must be developed to determine the changes in REE during antineoplastic treatments considering the factors influencing REE and TEE to determine the clinical and nutritional situations when ESPEN recommendations had the best agreement in population with cancer.