INTRODUCTION
Gastric cancer (GC) is one of the most common causes of cancer-related mortality worldwide. Globally, GC is the fourth leading cause of cancer mortality in males and females in 2020, based on global cancer statistics produced by the International Agency for Research on Cancer (1). GCs are primarily classified into two topographic subsites: cardia GC and non-cardia GC (2,3). The cardia GC arises in the region closest to the esophageal-gastric junction, and thus, share epidemiological features with esophageal cancer. The non-cardia GC arises in the distal portions of the stomach (2-4). These two subsites of GC were more prevalent in Eastern/Southeastern Asia countries. Men had higher rates than women for both GC subsites but specifically for cardia GC (male-to-female ratio of 3:1). Even though the incidence of non-cardia cancer has been dropping over the last years in most countries, but the incidences of cardia GC have remained constant or increased (5).
GC is a heterogeneous malignant disease and numerous factors are involved in its pathogenesis (6). Major GC risk factors include environmental factors like age, Helicobacter pylori (H. pylori) and Epstein-Barr virus (EBV) infection, race, sex, obesity, gastroesophageal reflux, tobacco, alcohol, diet, and genetic factors (6-8). The environmental factors like diet and lifestyle may positively or negatively affect the pathogenesis of GC (9-11), thus modifying these factors may control the risk of GC and reduce its incidence rate.
Epidemiological studies that examined dietary intake and plasma levels of various micronutrients and GC have reported inconsistent results (12,13). Vitamin C, vitamin E, retinol, and selected carotenoids have protective effects against gastric carcinogenesis (11,14,15). A few case-control studies showed that high intake of folate was inversely associated with the risk of GC (12,16,17), but findings from another case-control study did not support that finding (13). Data on the effect of intake of other minerals on the risk of GC is limited and generally inconclusive (13,18,19). Given the observed different results in the previous studies, the present study aimed to investigate the association between micronutrients intake and risk of GC in a case-control study conducted in Jordan.
MATERIALS AND METHODS
STUDY DESIGN AND PARTICIPANTS
This case-control study was performed in Jordan from March 2015 to August 2018. A total of 173 GC cases and 313 controls agreed to participate in this study. The cases were recruited from King Hussein Cancer Center, oncology clinics at King Abdullah University Hospital, Jordan University Hospital, and Al-Bashir Hospital. The controls were community based and they were hospital workers, patient visitors, patient escorts, university students, and university workers. The controls were comparable to the cases accordance with sex, age, occupation, and marital status. The proportion of cases to controls in this study was 1:1.
Inclusion criteria consist of being Jordanian aged ≥ 18 years, able to communicate verbally, and free of any chronic diseases that require dietary interventions. Individuals who were critically ill, unable to communicate verbally, pregnant or breastfeeding mothers, and diagnosed with cancer for more than six months or being affected by GC as a second cancer were excluded from this study.
The study protocol was approved by the Institutional Review Board Ethics Committee of the King Hussein Cancer Center (IRB No. 15 KHCC 03, Amman, Jordan), King Abdullah University Hospital, Jordan University Hospital, and Al-Bashir Hospital. Prior to participation in this study, all participants were asked to give a signed written informed consent. The study was conducted based on the Declaration of Helsinki.
DATA COLLECTION
Demographic, health, physical activity, and dietary information was collected by trained nutritionists using structured questionnaires. Personal questionnaire includes data about age, gender, marital status, education, occupation, family income/month, smoking, family history of GC, and history of stomachache and stomach ulcers.
ANTHROPOMETRIC MEASUREMENTS
Trained Nutritionists measured body weight and height for all study participants using standardized techniques and calibrated scales. Body weight was measured to the nearest 0.1 kg using a calibrated scale (Seca, Germany) while participants wore minimal clothing and without shoes, Height was measured to the nearest 0.1 cm with the participant in the full standing position without shoes using a calibrated stadiometer (Seca, Germany). Body mass index (BMI) was computed as weight in kilograms divided by height in squared meters (20).
PHYSICAL ACTIVITY QUESTIONNAIRE
The physical activity level of the study participants was assessed using a validated seven-day Physical Activity Recall (PAR) questionnaire. The seven-Day PAR is a structured and validated questionnaire that relies on the participant’s recall of frequency, intensity, time, and type of physical activity performed over a seven-day period (21). Daily number of hours expended in sleeping and different physical activity levels were taken and transformed into metabolic equivalents (MET). Based on the scoring instructions, sleeping was assigned a value of 1.0 MET, light activity a value of 1.5 METs, moderate activity a value of 4.0 METs, and for a very hard activity a value of 7.0 METs or greater (22). Physical activity was computed as the time (minutes) spent to each activity multiplied by the equivalent MET for that activity and multiplied again by the number of days that the activity was undertaken per week period, as stated in the following equation: (MET level x minutes of activity/day x days per week) (21).
DIETARY ASSESSMENT
Dietary intake of micronutrients was assessed by a validated Arabic quantitative FFQ (23). The FFQ questions examined the data dealing with the participant’s dietary history. The FFQ assessed intake of 112 food items: 21 items of vegetables; 21 items of fruits and juices; 17 items of meat such as red meat; 12 items of cereals and legumes; nine items of milk and dairy products; four items of soups and sauces; five items of drinks; nine items of snacks and sweets; and 14 items of herbs and spices. The study participants were interviewed face-to-face by trained nutritionists and asked the participants to recall how frequently, on average, during the last year they had consumed one standard serving of specific food items in 10 classes (1-6 times/year 7-11 times/year, 1 time/month, 2-3 times/month, 1 time/week, 2 times/week, 3-4 times/week, 5-6 times/week, 1 day, 2 or more/day). The portion sizes of each food item were categorized according to commonly used portion sizes into small, medium, and large. Food models and standard measuring tools were used to help participants in determining the consumed portion size of foods precisely. Dietary analysis software (Food Processor SQL version 10.1.129; ESHA, Salem, OR, USA) was used to estimate dietary micronutrients intake, however, that software was modified to include nutritional data about Jordanian cuisine (24).
STATISTICAL ANALYSIS
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 28 (IBM Corporation, Armonk, NY, USA). The statistically significant level was placed at p < 0.05. Data was represented as mean ± standard error of mean (SEM) for normally distributed continuous variables Independent Sample t-test was used to detect differences in normally distributed continuous variables between GC group and control group. Frequencies and percentages were calculated to describe categorical variables were represented as frequencies and percentages. Pearson chi-square was used to find differences in sociodemographic characteristics such as marital status, education, and employment status, and health characteristics such as smoking family history of GC, and personal history of stomachache and stomach ulcer between GC group and control group.
Median (33.3rd-66.7th percentile) was used for non- normally distributed data (micronutrients intake). Mann-Whitney U test was used to identify differences in intakes of micronutrients between GC cases and controls.
Intakes of micronutrients were modeled using tertiles of distribution in the study participants with the first tertile being the lowest intake and the third tertile the highest. Odds ratios (ORs) and their corresponding 95 % confidence intervals (95 % CIs) of GC according to different tertiles of daily micronutrient intake were calculated using a multinomial logistic regression model. All models were adjusted for all potential cofactors like total caloric intake, age, gender, marital status, education, BMI, smoking, period of smoking, family history of gastric cancer, personal history of gastric ulcer, and physical activity (MET-min/week) which were reported as risk factors of GC in previous studies (25-27). Linear logistic regression model was used to compute p-value for trend.
RESULTS
Table I gives distribution of 173 GC cases and 313 controls according to gender, anthropometric measurements, socio-demographic and health characteristics. No differences were seen between cases and controls in age, height, marital status, and medical history of chronic diseases. Control males were significantly more active than GC males (3406.6 ± 222.4 vs. 1851.6 ± 192.8, p < 0.001). Pre-diagnosis weight and BMI were significantly higher in GC males compared to control males (p < 0.05), whereas current weight and BMI were significantly lower in cases of both sexes (p < 0.001). The participants with a family history (beyond the second degree) of GC was significantly more frequent in GC group compared to the controls (p < 0.001). GC cases had a higher incidence of gastric ulcers and gastric ache than the control group (p < 0.001). GC patients consumed more energy than controls (p < 0.001).
Table I. Socio-demographic and health characteristics of 173 gastric cancer cases and 313 controls based on gender.

*p values calculated by sample t-test for continuous variables and Pearson Chi-square for categorical variables.
p value < 0.05 was considered statistically significant.
**MET: metabolic equivalents-minutes/week.
Table II displays the median daily intakes, the 33.3rd, and 66.7th percentile of micronutrients of GC cases and controls based on gender. The consumption of vitamin A, beta-carotene, vitamins B1, B2, B3, B6, B12, C, D, E, folate, calcium, chromium, copper, iodine, iron, magnesium, phosphate, potassium, selenium, sodium, and zinc was significantly higher in GC males compared to control males (p < 0.05), except fluoride. However, the GC females had significantly higher intakes of vitamin A, vitamins B1, B3, C, E, folate, calcium, chromium, copper, iodine, iron, magnesium, potassium, and zinc when compared to the control females (p < 0.05).
Table II. Micronutrients intake of 173 gastric cancer cases and 313 controls participating in a case-control study.
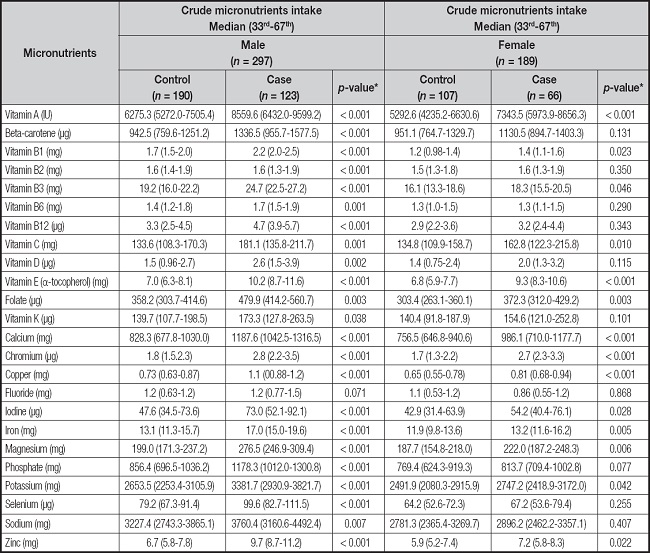
*p values were calculated by Mann-Whitney U test and p value < 0.05 was considered statistically significant.
Table III shows the association between intake of selected vitamins and the risk of GC. There were significant negative linear trends for the risk of GC with increasing consumption of vitamins A, E, K, beta-carotene, and folate. Compared to the lowest tertile, the highest intakes of vitamin A (OR, 0.26; 95 % Cl 0.12-0.57, p-value for trend = 0.001); beta-carotene (OR, 0.42; 95 % CI, 0.20-0.86, p-value for trend = 0.019); vitamin D (OR, 0.47; 95 % CI, 0.23-0.95, p-value for trend = 0.031), vitamin E (OR, 0.06; 95 % CI 0.02-0.18, p-value for trend < 0.001), folate (OR, 0.39; 95 % CI 0.19-0.79, p-value for trend < 0.001), and vitamin K (OR, 0.40; 95 % CI 0.20-0.80, p-value for trend = 0.006) were inversely associated with risk of GC. Statistically significant protective effect was observed for moderate consumption (T2 vs. T1) of vitamin B2 (OR, 0.36; 95 % CI, 0.18-0.75, p-value for trend < 0.001), vitamin B3 (OR, 0.28; 95 % CI, 0.12-0.62, p-value for trend < 0.001), vitamin B6 (OR, 0.29; 95 % CI, 0.13-0.60, p-value of trend < 0.001), vitamin B12 (OR, 0.48; 95 % CI, 0.24-0.96, p-value for trend < 0.001), and vitamin C (OR, 0.34; 95 % CI, 0.15-0.65, p-value for trend < 0.001) on GC risk.
Table III. Adjusted odds ratios (ORs) and corresponding 95 % confidence intervals (CIs) of association of intake of selected vitamins and risk of gastric cancer among Jordanian.

aAdjusted for caloric intake, age, gender, marital status, education, BMI, smoking, period of smoking, family history of gastric cancer, medical history of gastric ulcer, and physical activity. The control group was considered the reference group for analysis.
bReference tertile.
The adjusted ORs of GC for different tertiles of intake of vitamins categorized based on gender are presented in table IV. There were significant inverse association between GC risk and high dietary intake of vitamin A (OR, 0.12; 95 % CI, 0.04-0.36, p-value for trend < 0.001); beta-carotene (OR, 0.35; 95 % CI, 0.13-0.92, p-value for trend = 0.038); vitamin B1 (OR, 0.17; 95 % CI, 0.05-0.59, p-value for trend = 0.006), vitamin B3 (OR, 0.13; 95 % CI 0.03-0.46, p-value for trend = 0.002), vitamin B12 (OR, 0.25; 95 % CI 0.08-0.75, p-value for trend = 0.013), folate (OR, 0.12; 95 % CI 0.03-0.42, p-value for trend = 0.033), and vitamin K (OR, 0.39; 95 % CI 0.15-0.98, p-value for trend = 0.038) in male group. In contrast, the higher dietary vitamin C intake (OR the highest vs. the lowest tertile = 0.27, 95 % CI: 0.00-0.08, p-value for trend = 0.038) was associated with reduction of GC in female group. Negative relationships were revealed for moderate dietary intakes of vitamin B1 (OR 0.13; 95 % CI: 0.04-0.46, p-value for trend = 0.033), vitamin B6 (OR 0.23; 95 % CI: 0.07-0.75, p-value for trend = 0.001), vitamin C (OR 0.27; 95 % CI: 0.08-0.9, p-value for trend = 0.033), and folate (OR 0.27; 95 % CI: 0.09-0.85, p-value for trend < 0.001) in female group.
Table IV. Adjusted odds ratios (ORs) and corresponding 95 % confidence intervals (CIs) of association of intake of selected vitamins among Jordanian based on gender.
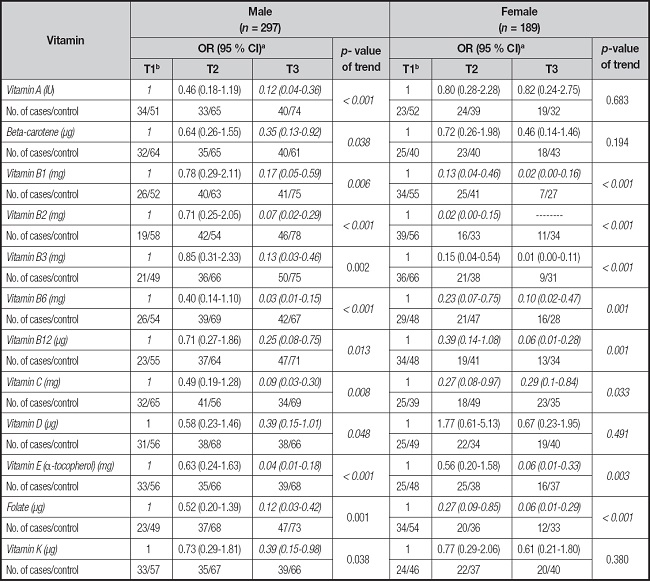
aAdjusted for caloric intake, age, marital status, education, BMI, smoking, period of smoking, family history of gastric cancer, personal history of gastric ulcer, and physical activity. The control group was considered the reference group for analysis.
bReference tertile.
Table V gives adjusted ORs of GC for the highest versus the lowest tertile of intake of selected minerals. Comparing the highest tertile to the lowest tertile, odds of GC risk reduced significantly for intake of chromium (OR, 0.26; 95 % CI, 0.12-0.57, p-value for trend < 0.001), iodine (OR, 0.42; 95 % CI 0.20-0.86, p-value for trend = 0.033) and selenium (OR, 0.17; 95 % CI 0.07-0.42, p-value for trend < 0.001). The protective effect was also found for moderate consumption as the middle tertile compared to the lowest tertile of calcium (OR, 0.42; 95 % CI, 0.20-0.86, p-value for trend < 0.001), copper (OR, 0.21; 95 % CI, 0.10-0.45, p-value for trend < 0.001), iron (OR, 0.38; 95 % CI, 0.19-0.82, p-value for trend = 0.019), magnesium (OR, 0.15; 95 % CI, 0.07-0.34, p-value for trend < 0.001), phosphate (OR, 0.27; 95 % CI, 0.12-0.58, p-value for trend < 0.001), sodium (OR, 0.24.; 95 % CI, 0.11-0.54, p-value for trend < 0.001), and zinc (OR, 0.32; 95 % CI, 0.15-0.68, p-value for trend < 0.001) on GC risk. However, the consumption of fluoride and potassium showed null associations with risk of GC.
Table V. Adjusted odds ratios (ORs) and corresponding 95 % confidence intervals (CIs) of association of intake of selected minerals among 173 gastric cancer cases and 313 controls participating in a case-control study.
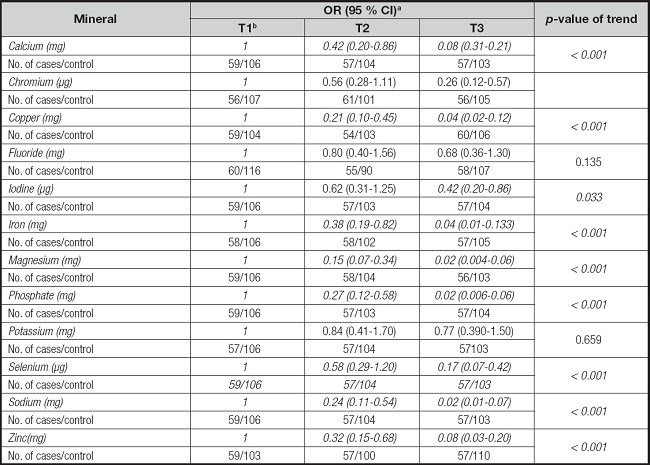
aAdjusted for caloric intake, age, gender, marital status, education, BMI, smoking, period of smoking, family history of gastric cancer, personal history of gastric ulcer, and physical activity. The control group was considered the reference group for analysis.
bReference tertile.
Table VI shows the relationships between GC and intake of selected minerals classified based on gender. GC was negatively associated with chromium, with an OR of 0.26 (95 % CI = 0.09-0.70) for the middle, iron, with an OR 0.06 (95 % CI = 0.01- 0.25) for the middle, and magnesium, with an OR of 0.29 (95 % CI = 0.11-0.83) for the middle versus lowest tertile of intake in male group. Likewise, the high consumption of calcium, chromium, copper, magnesium, phosphate, potassium, selenium, sodium and zinc was inversely associated with GC in males. Whereas great reduction in risk of GC was seen in females who consumed calcium (OR the middle vs. the lowest tertile = 0.32, 95 % CI: 0.11-0.97, p-value for trend < 0.001) and selenium (OR the middle vs. the lowest tertile = 0.31, 95 % CI: 0.11-0.87, p-value for trend = 0.001). The moderate and high intakes of calcium, iron, magnesium, phosphate, selenium, and sodium were inversely associated with GC in females (p < 0.05). An inverse relationship was detected for highest intake of zinc (OR = 0.03, 95 % CI: 0.00-0.15, p-value for trend < 0.001).
Table VI. Adjusted odds ratios (ORs) and corresponding 95 % confidence intervals (CIs) of association of intake of selected minerals among based on gender.
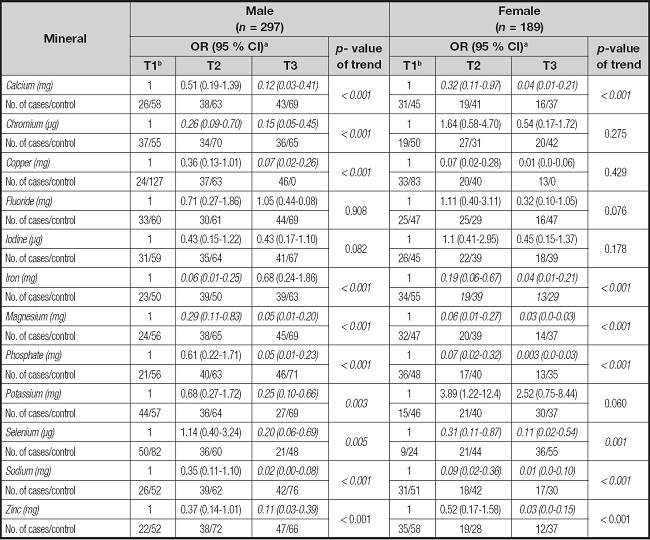
aAdjusted for caloric intake, age, marital status, education, BMI, smoking, period of smoking, family history of gastric cancer, personal history of gastric ulcer, and physical activity. The control group was considered the reference group for analysis.
bReference tertile.
DISCUSSION
The association between the consumption of a healthy diet that is rich in fruits and vegetables and GC is previously documented; however, the evidence is controversial regarding the association between micronutrients and GC risk (28). Here, we observed several significant favorable effects of micronutrients against the risk of GC.
Lunet et al. (28) found no association between vitamin C, E, or carotenoids with the risk of GC; however, these results were adjusted for H. pylori infection which has a causal association with GC (28). Other studies reported protective effects of the antioxidant vitamins; A, C, and E on gastric carcinogenesis (29-31). This effect of antioxidant vitamins is confirmed by a systemic review and a meta-analysis of prospective and well-designed observational studies and relevant evidence (11,32). These vitamins may modify the pathogenesis of GC by their antioxidant capacity, free-radical scavenging capacity, and by reducing the risk of H. pylori infection (29,33). Specifically, vitamin C inhibits the conversion of nitrites to carcinogenic N-nitroso compounds; therefore, protects gastric mucosa (29,33,34).
The association between gastric carcinogenesis and vitamin D is controversial. Neither dietary vitamin D consumption nor vitamin D-related genes modulated GC risk in the Korean population (35). Although a systematic review of evidence found no association between GC risk and dietary vitamin D or its serum level (36), other case-control and cross-sectional studies, including the current study, found a beneficial effect of vitamin D on GC risk (12,37). Yet, vitamin D has an anticancer activity against various cancers, and it has been documented to reduce the risk of H. pylori infection (35,38). To the best of our knowledge, no previous study reported the association between dietary vitamin K and GC risk. Vitamin K is associated with the consumption of plant-based foods that are rich in many other nutrients and non-nutrients components which could underly the protective effect of high vitamin K consumption observed in this study.
An Italian case-control study found no significant relationships between GC risk and the intake of iron, calcium, potassium, and zinc (13). Another hospital-based study showed inverse associations between GC and consumption of potassium and iron (18). Sodium intake has been related to an increased risk of GC in several epidemiological studies (13,18,39). Conversely, we detected a negative GC risk with moderate consumption of sodium and no association with higher consumption. Also, our results did not show any significant association with potassium intake. The negative impact of high sodium intake on GC incidence has been previously documented in one study instead of other studies (28). High dietary sodium irritates gastric mucosa, induces its inflammation and damage, incites H. pylori infection, and increases the risk of gastric carcinogens (34). However, here, the median daily intake of sodium for cases and control was found to be less than 3500 mg per day which is not considered high compared to Western, European, and East Asian countries where sodium consumption may reach up to 13 g per day (40,41).
Our results suggest a protective effect of high consumption of B vitamins on the risk of GC. The association between dietary intake of B vitamins and GC is debatable. One-carbon metabolism nutrients (folate, vitamin B6, vitamin B2, and vitamin B12) are essential for DNA methylation, and insufficient intake of these nutrients potentially could encourage carcinogenesis (42,43). Thiamine and niacin are coenzymes in several cellular functions (43). Thiamine may affect cancer risk through several mechanism such as protein expression, oxidative stress, inflammation, and cellular metabolism (44), while niacin may influence cancer risk through its antioxidant activity (18). The current evidence is not consistent on the link between GC risk with the consumption of folate, vitamin B6, or vitamin B12 (43,44,45). Similarly, the associations of thiamine/niacin and cancer risk is yet to be defined (18,46,47). However, Miratni et al. (42) found that low serum concentrations of vitamin B12 augmented the risk of GC by 5.8 folds. Thus, the association could be obvious when habitual intake is not sufficient to support health. Also, several factors may interfere with the bioavailability of B vitamins including smoking, alcohol intake, some medications, as well as gut microbiota. Well-nourished individuals may not benefit from high consumption of B vitamins to reduce the risk of gastric carcinogenesis (43).
Evidence regarding the effect of dietary minerals on the incidence of GC is scarce and inconsistent (49,50,51). To our knowledge, no available evidence reported the effect of dietary fluoride, copper, or chromium on GC. Nevertheless, consistent with our results, gastric carcinogenesis might be suppressed by high dietary intake of some minerals including selenium and zinc which play essential roles in oxygen species-scavenging activity (51,52,53); magnesium and calcium which are required for overall homeostasis and health (54); and iron (53) where its low serum levels and a higher rate of iron deficiency anemia were found to augment GC risk (55-57). The latter evidence indicates the influence of mineral status on the risk of GC where iron deficiency anemia could increase DNA damage and oxidative stress (56). Another evidence suggested a higher risk of GC in case of a high-iron diet, where it is suggested to induce tumorigenesis by the effect of dysregulated iron metabolism on iron-modulated function (58). Also, different dietary sources may have variable impacts on disease risk, for example, heme iron was found to be associated with a higher risk for GC which could be provoked by other components or processing methods (48). Concerning iodine, a protective effect on GC of high dietary iodine was observed, which is consistent with a previous report of a high incidence of goiter among GC patients and is supported by the antioxidant activity of iodine (59). Previous studies showed lower intake of phosphorus among GC patients (13,60), here, we found protective effects against GC of moderate consumption of phosphorus.
While several other factors may contribute to the risk of GC such as H. pylori infection, host susceptibility, environmental exposures, and other dietary components such as phytochemicals and fat, the findings of this study may aid in increasing awareness of population and health care members toward the avoidance or encouragement of the consumption of specific micronutrients.
STUDY STRENGTHS AND LIMITATIONS
This case-control study has several strengths including the adjustment of statistical analyses for many substantial confounders is believed to strengthen our findings by eliminating the effects of these variables on GC risk. Compliance with the questionnaire was high with an eminent response rate of > 95 %. On the other hand, it is worth mentioning the limitations of this research including some questionnaire-inherited limitations that cannot be excluded; nevertheless, these factors were attenuated by precautionary measures. The accuracy of dietary recall tools is affected by possible recall bias and estimation errors; however, their effects were lessened using a validated FFQ along with food models and measuring tools to enhance the accuracy of portion size estimation. The used FFQ includes cultural foods, commonly used portion sizes for each food item, and accounted for seasonal variations for a more precise estimation of participants’ dietary history. Also, differential recall bias may have influenced the dietary data due to knowledge of the disease status as well as due to the possibility that cases might have changed diet due to their disease or corresponding symptoms; yet, the inclusion of recently diagnosed patients could have reduced such bias. Another limitation is the lack of biochemical levels of these micronutrients; however, further research is warranted to investigate the effect of their serum levels on the pathogenesis of GC. Also, the interviewers were not blinded for the diagnosis of the participants (i.e., cases and control); nevertheless, all interviewers were well-trained and treated the participants professionally and identically, regardless of their case and control status. Moreover, the body composition of the study participants was not measured, however, the body composition is an important factor when it comes to investigate correlation between dietary factors and cancer due to most cancer patients have wasting, which includes muscle loss whether or not there is also fat loss.
CONCLUSION
The findings of this study support the advantageous effects of moderate consumption of micronutrients on GC risk which can be achieved by a balanced, healthy diet. GC was inversely associated with the consumption of vitamin A, beta-carotene, vitamins D, E, K, B2, B3, B6, B12, and C, folate, chromium, iodine and selenium. Additionally, a protective effect was observed for consumption of calcium, copper, iron, magnesium, phosphate, sodium, and zinc. Increasing the population’s awareness toward the health benefits of micronutrients and regarding their dietary sources would reduce GC-related morbidity and mortality. Further research is required to confirm our findings and elucidate the underlying mechanisms of these associations.














