INTRODUCTION
Since December 2019, when the first cases of pneumonia were reported in Wuhan, China, several studies have been conducted to identify the causative agent, SARS-CoV-2. This virus has a high transmission capacity and causes the disease known as COVID-19, which can range from mild symptoms (cough, fever) managed on an outpatient basis to severe cases (respiratory distress, septic shock) requiring hospitalization.
The COVID-19 pandemic, declared by the World Health Organization on March 30, 2020, has affected millions of people worldwide, with a high number of confirmed cases and unfortunately, a significant number of fatalities. Clinical risk factors for a fatal outcome associated with SARS-CoV-2 include acute kidney injury, diabetes, hypertension, cardiovascular diseases, cancer, elevated D-dimer, male sex, advanced age, smoking, and obesity (1,2).
Chronic kidney disease (CKD) is a common condition that can have serious consequences and is defined as the presence of structural or functional kidney abnormalities for at least three months. After diagnostic confirmation, CKD is classified into 5 stages based on glomerular filtration rate (GFR) in ml/min, ranging from G1 with GFR > 90 ml/min, considered as a mildly decreased kidney function, to G5 < 15 ml/min, classified as kidney failure. The ENRICA-Renal study has reported a prevalence of CKD of 15 %, affecting more males and increasing with age. In advanced stages of CKD, renal replacement therapy (RRT) is necessary. The most recommended treatment is kidney transplantation, but in many cases, it is not feasible, so hemodialysis (HD) is performed more frequently. In Spain, the O.N.T./S.E.N. Registry shows that in the last ten years, the number of new patients requiring RRT has increased from 140 per million population in 2020 to 149.5 per million population in 2021. The registry also reports high mortality rates, with an annual rate of 13 % for HD patients (3-5).
Since the beginning of the pandemic, patients on HD have been considered high-risk. COVID-19 can lead to more severe complications and worse outcomes in patients undergoing chronic HD treatment (6-8). The mortality rate related to SARS-CoV-2 pneumonia in patients with CKD is 14 to 16 times higher than in the general population. The initial mortality rate in HD of 23 % has been decreasing thanks to science (9).
Nutritional status changes and affects the well-being and survival of patients undergoing chronic HD treatment. Therefore, it is crucial to consider integrated and objective tools like body composition because the body mass index (BMI) used in the general population does not provide complete information for these patients. It has been shown that a significant increase in body fat mass during the first year, especially in those with poor nutritional status, promotes sarcopenic obesity (10,11).
Obesity is a chronic inflammatory state that contributes to metabolic disorders such as diabetes and dyslipidemia. Obesity is also a nutritional factor that increases the risk of SARS-CoV-2 infection (12). Additionally, the accumulation of fat in the visceral compartment (13) is metabolically more active and is associated with metabolic abnormalities and inflammation, making it considered a risk factor for cardiovascular disease and mortality (14). Several studies also show that nearly 50 % of individuals over 65 years of age on HD may suffer from malnutrition and loss of muscle mass (15,16). Malnutrition associated with inflammation was linked to increased mortality in patients on HD (17). It is known that in this population obesity and hypercholesterolemia can have a protective effect and, paradoxically, be associated with better outcomes. This result has been called “reverse epidemiology” (13).
Therefore, it is unknown whether obesity could have a protective effect or be a risk factor for HD patients with COVID-19. Likewise, the role of other nutritional parameters of these patients on the prognosis of SARS-CoV-2 infection is unknown.
The objective of this study is to describe the nutritional characteristics and their potential association with the prognosis of COVID-19 in patients undergoing chronic HD treatment.
METHODS
This was a descriptive retrospective, observational design. The inclusion criteria were patients with ACKD undergoing chronic treatment in the Hemodialysis Unit of the hospital who suffered from COVID-19 from the beginning of the pandemic (11/3/2020) until the start of vaccination against SARS-CoV-2 (15/4/2021). The diagnosis of COVID-19 was made using:
− Antigen + or PCR + for SARS-CoV-2 in nasopharyngeal exudate
− Serum Immunoglobulin A or G anti SARS-CoV-2 and clinical syndrome compatible with COVID-19. This last assumption was only maintained during the first wave, March to May 2020, in which the health crisis was accompanied by a lack of diagnostic reactive means.
The exclusion criteria were not being on HD treatment or not meeting the described COVID-19 diagnostic criteria or receiving the SARS-CoV-2 vaccine. Data sources included electronic medical records and the Nefrolink software.
The following baseline descriptive variables were recorded: age (years), gender (female/male), residence (home-institution), comorbidity by Charlson index, diabetes mellitus (yes/no), respiratory failure (yes/no), congestive heart failure (yes/no), chronic obstructive pulmonary disease (yes/no), vascular calcifications (yes/no), duration of treatment in hemodialysis (months), acute phase reactants (procalcitonin (PCT), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), neutrophilic leukocytosis (NL), vascular access for dialysis such as arteriovenous fistula (AVF) or central venous catheter (CVC), dialysis session duration (minutes) as average of the 6 month prior to the contagion of COVID-19, and dialysis dose measured by single pool Kt/V from the most recent monthly control before infection. The dialysis dose was measured by second-generation KtV Daugirdas (18): Kt/V Daug = - ln ((C2 / C1) - (0.008 * T)) + (4 - 3.5 * (C2 / C1)) * UF / P (C1: pre-dialysis blood urea in mg/dL; C2: post-dialysis blood urea in mg/dL; T: session duration time in minutes; UF: volume of removed ultrafiltrate in liters; P: post-dialysis weight of a subject in kilograms), and Kt/V residual by Kt/V residual = (5,9 x KRU) / Volume (KRU: residual urea clearance).
Nutritional control variables were also recorded based on the latest measurement prior to contracting COVID-19. The recorded serum levels included albumin (g/dL), triglycerides (TG) (mg/dL), LDL cholesterol (mg/dL), ferritin (ng/mL), transferrin (mg/dL), transferrin saturation index (TSI) (%), parathyroid hormone PTHc (pg/mL), calcium (millimol/L), phosphorus (mg/dL), vitamin D (ng/mL), folic acid (ng/mL), pH, HCO3 (mEq/L), PCR (mg/dL) creatinine (mg/dL), and urea (mg/dL). Baseline body composition parameters were also recorded prior to illness, including body mass index (BMI) (kg/m2), fatty tissue index (FTI) (kg/m2), lean tissue index (LTI) (kg/m2), extracellular/intracellular water, and phase angle (degree) (°). Body composition was determined using multifrequency bioimpedance analysis with BCM Fresenius stereoscopic equipment, based on the resistance or opposition to the passage of an applied electric current (19). The evolution of weight change in the last year was calculated as final weight (at the time of infection) minus initial weight (one year prior to infection) (20).
For the choice of variables, the recommendations of the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) were followed, considering those available as it was a retrospective study. Also assessing that there are previous studies that have demonstrated its prognostic value both for nutritional deterioration and for the relationship between body composition and mortality in HD patients. Other authors have reported the usefulness of variables such as albumin, creatinine, urea, cholesterol, C-reactive protein, adequacy of dialysis (with reference to Kt/V) and weight change as predictors of nutritional deterioration, hospitalization or death (21,22).
The severity of the disease COVID-19 was classified according to the WHO as critical (requires life sustaining treatment, acute respiratory distress syndrome, sepsis, septic shock), severe (oxygen saturation < 90 % in ambient air, pneumonia, severe respiratory distress) or non-severe (absence of signs of serious or critical illness) (5). The days of hospital admission were recorded if there were any.
The patients were divided into two groups based on their vital prognosis: the exitus group, which included patients who passed away during the acute phase of COVID-19, and the survivor group, consisting of patients who survived.
SAMPLE SIZE
Assuming an alpha risk of 0.05 and a beta risk of 0.2 in a bilateral test, 17 subjects were required in the first group and 3 subjects in the second group to detect a difference equal to or greater than 10 units of body mass index (BMI). It is assumed that the common standard deviation is 5, and a follow-up loss rate of 30 % was considered. The sample size calculation was performed using the GRANMO sample size calculator Version 7.12 April 2012, from the Program of Research in Inflammatory and Cardiovascular Disorders, Institut Municipal d’Investigació Médica, Barcelona (Spain).
DATA PRESENTATION AND STATISTICAL ANALYSIS
Continuous variables are presented as mean and standard deviation, while categorical variables are presented as absolute value and corresponding frequency (%). Univariate analysis was conducted to study the relationship between exitus as the independent variable and the clinical characteristics of the patients, identifying markers that could predict or impact the prognosis of COVID-19. The Mann-Whitney/Wilcoxon rank-sum test was used for non-parametric unpaired continuous variables, and the Fisher’s exact test was used for non-parametric unpaired categorical variables. Subsequently, a binary logistic regression was performed with Exitus (yes/no) as the outcome variable and the variables age, sex, BMI, TG, FTI, LTI, Ferritin, and TSI as independent variables. The statistical analyses were conducted using software packages available for this type of study, such as “R” version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
ETHICAL CONSIDERATIONS
The study was authorized by the Ethics Committee for Research with Medicinal Products of the CEIM Hospital Universitario y Politécnico La Fe, and the Research Ethics Committee of the Universidad de Murcia. The handling of patient data, generated during the course of the research project, complied with Regulation (EU) 2016/679 of the European Parliament and the Council of 27 April 2016 on Data Protection (GDPR) and Organic Law 3/2018 of 5 December on the Protection of Personal Data and guarantee of digital rights. In the patient database, personal identification data were dissociated from clinical care data. This is a retrospective study without intervention on the patient and with data protection, therefore the informed consent of the patients was waived.
RESULTS
Between March 2020 and April 2021, 189 patients received chronic HD treatment in the hospital unit. Of these, 22 patients were diagnosed with COVID-19 (12 %) in that period. Of the 22 patients affected by COVID-19, SARS-CoV-2 was diagnosed by a positive PCR in 19 patients, and by symptomatic disease with positive serology in 3 patients. Mean age (SD) was 71 (11.6) years, 10 were women, 2 were living in a nursing home and the rest in their family homes. The Charlson index was 6.59 (2.32) points, diabetes mellitus 10, HD vintage 51.6 (40.84) months; 2 patients had previously received a kidney transplant that was currently non-functioning, 16 had an arteriovenous fistula (AVF) as vascular access, and 6 had a central venous catheter (CVC). HD session time (minutes): mean dialysis session duration was 220.14 (23.4) minutes; baseline Kt/V single pool dose was 1.7 (0.39) and residual kt/v was 1.07 (0.90). It was observed that only 4 patients retained residual renal function, therefore the analysis was not relevant.
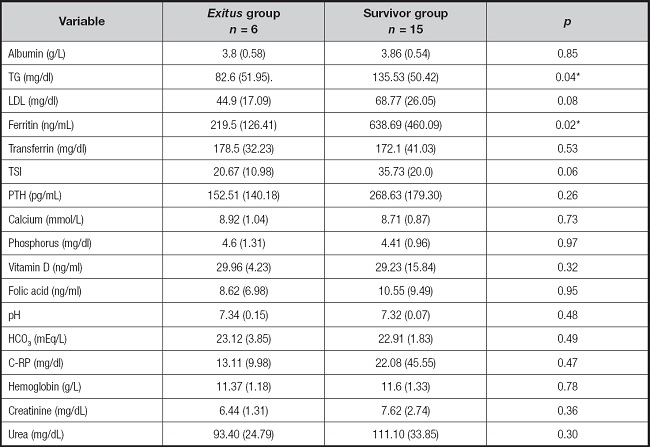
The evolution of COVID-19 patient disease is showed in table I.
Table I. Evolution of COVID-19 in HD patients.
Quantitative variables in mean (SD) and qualitative variables in absolute value (%).
*Acute phase reactants: procalcitonin, C-reactive protein, erythrocyte sedimentation rate, neutrophilic leukocytosis.
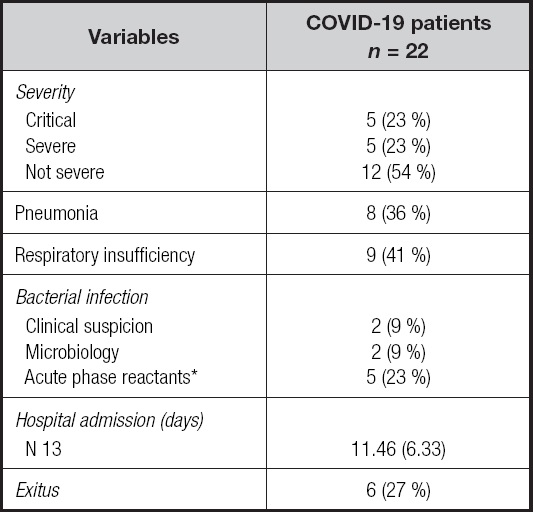
The different variables of patients with worse prognosis, exitus, were analyzed in comparison to those who survived, survivor. The general characteristics by group are shown in table II.
Table II. Patient characteristics by group.
Quantitative variables in mean (SD) and qualitative variables in absolute value (%). AVF: arteriovenous fistula; CVC: central venous catheter; COPD: chronic obstructive pulmonary disease; K: dialyzer clearance of urea; t: dialysis time; V: urea volume of distribution, approximately equal to total body water.
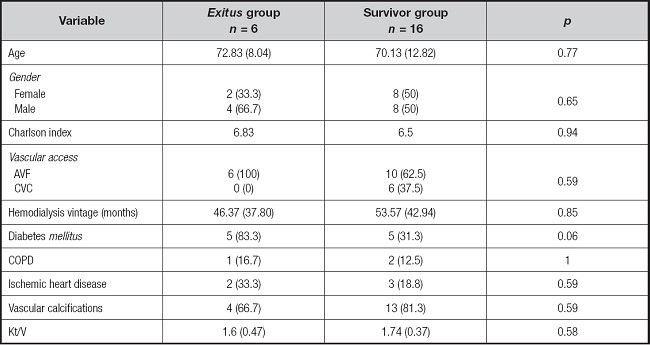
Of the 22 included patients, 16 had undergone body composition measurements (BCM) 6 months prior to the infection, and 6 months after the infection. The differences in body composition were analyzed between the exitus group and the survivor group. The results are shown in table III.
Table III. Body composition by group.
*Significant differences with p < 0.05 were observed.
Variables are presented as mean (SD). BMI: body mass index; FTI: fat tissue index; LTI: lean tissue index.
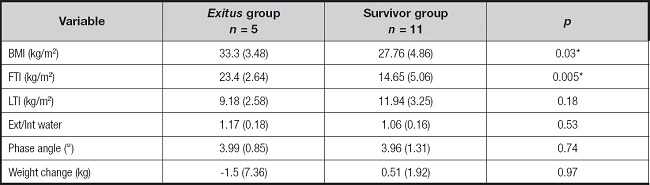
Differences between groups in serum levels of various variables related to patient’s nutritional status were analyzed. The results are shown in table IV.
DISCUSSION
In the present study, we analyzed the nutritional characteristics of patients undergoing chronic HD treatment diagnosed with COVID-19 prior to vaccination. The results demonstrate that higher body mass index and fat mass index, lower baseline levels of triglycerides and ferritin are associated with increased mortality.
To date, no author has described the relationship between nutritional parameters and COVID-19 mortality in HD patients. The results of the present study remind us of the importance of diagnosis and adequate nutritional management in these patients. The implementation of such measures could reduce mortality in HD patients who become infected with SARS-CoV-2.
Eliminating CKD as a risk factor would decrease the percentage of the world’s population at highest risk of severe COVID-19 from 22 % to 17 %. CKD therefore explains the increased risk of severe COVID-19 for approximately one in four high-risk people worldwide (23). In another systematic review of HD patients, Parra-Martos et al. (24) demonstrated that the mortality rate in this group of patients ranged between 20 % and 30 %, with risk factors being advanced age, multimorbidity, clinical fragility or weakness, male sex, and critical presentation of the disease, which is also reflected in the study by Jager-Kitty et al. (25). In our study the mortality rate of patients on HD also exceeds 20 % and is higher in men.
The findings reported in this study are consistent with others conducted in the general population, which show a relationship between higher body mass index and adipose tissue and mortality from COVID-19 (26).
However, there are also numerous studies reporting the phenomenon of “the obesity paradox in HD patients.” According to Kamyar Kalantar-Zadeh et al. (27), their results showed that obese patients had lower mortality compared to those with normal weight or underweight.
Additionally, Nirupama Ramkumar et al. (28) investigated the association between obesity and inflammation in patients with CKD, including those on HD. The results indicated that obesity was associated with lower levels of inflammatory markers, which could contribute to the observed higher survival in obese patients on HD. In a meta-analysis by Kamyar Kalantar-Zadeh et al. (29), they evaluated the association between obesity and mortality in hemodialysis patients. The results indicated an inverse relationship between BMI and mortality, supporting the existence of the “obesity paradox” in this population.
In the present study we observed that a higher BMI was associated with increased mortality from COVID-19. Obesity is a common nutritional disorder in advanced CKD, affecting between 20 % and 60 % of patients, and it is associated with higher overall comorbidity and particularly cardiovascular comorbidity. Central fat distribution is more sensitive to lipolytic stimuli and is accompanied by a pathological lipid profile (excess triglycerides and VLDL), hyperinsulinism, and peripheral resistance to insulin action, known as metabolic syndrome. These factors contribute to a chronic inflammatory state, oxidative stress, and impaired immune response, worsening the clinical course of COVID-19 (30).
In our study, better outcomes were observed in patients with higher baseline levels of ferritin and triglycerides, which could be attributed to a better baseline nutritional status compared to those who died. It is important to differentiate the interpretation of these determinations at baseline, related to nutrition, from the determination during the disease, related to inflammation. These determinations during the disease can be altered by the presence of inflammation and become markers of poor prognosis. Jahnavi Daru et al. (31), investigated the utility of serum ferritin as a marker of iron status in the presence of inflammation. It was found that ferritin levels are affected by inflammation and may not accurately reflect iron levels in the body, especially in cases of chronic inflammation. In a systematic review and meta-analysis by Karanvir Kaushal et al. (32), high levels of serum ferritin were associated with more severe disease and a negative/poor outcome in COVID-19. Therefore, serum ferritin level can serve as an important predictive biomarker in the management and triage of COVID-19 when determined during the disease. These studies provide preliminary evidence of the association between high levels of ferritin with an increased risk of unfavorable outcomes in patients with COVID-19.
Regarding the level of lipids, these may vary during the course of COVID-19, since lipidology is affected by both inflammation and organ damage caused by SARS-CoV-2 (33). In another study, Klaudia Kowalska et al. (34), focused on the impact of SARS-CoV-2 infection on lipid metabolism, showing a decrease in serum cholesterol levels, HDL-C, LDL-C, and abnormalities in triglycerides during the infection. There was a direct correlation between a decrease in these factors and the stage of the disease.
A noteworthy outcome from our investigation was the identification of a significant correlation between low baseline triglyceride levels and increased mortality in COVID-19-affected hemodialysis patients. This observation contributes to the ongoing discourse in the literature on lipid profiles in hemodialysis. While Gonzáles-Rubianes et al. (35) and Chang et al. (36) delve into various lipid aspects in hemodialysis, our emphasis on the connection between low triglyceride levels and mortality adds a nuanced perspective to this complex scenario. This contrasts with findings from studies like Xie et al. (37), which proposes an direct association between mortality and specific lipids in patients with kidney disease. The conflicting results underscore the intricacies of these relationships, emphasizing the necessity for further investigations to elucidate the distinct role of triglycerides in the context of COVID-19 among hemodialysis patients.
STUDY LIMITATIONS
This study has several limitations. Firstly, the sample size is small and requires further studies for confirmation. Additionally, being an observational, descriptive, and retrospective study, some analytical and body composition determinants were not available for all patients. The high risk of suffering from COVID-19 for HD patients marks the need to receive the vaccine. Therefore, at the time the study was proposed, it was not ethical to delay vaccination. The data had to be collected retrospectively. It would have been preferable to conduct a prospective study; however, for these reasons, the present study is retrospective with the known limitations of this methodology.
CONCLUSION
In chronic hemodialysis patients, the mortality rate for COVID-19 is high, exceeding 20 %. Both higher body mass index and fat mass index, lower baseline levels of triglycerides and ferritin are associated with increased mortality on COVID-19. The baseline nutritional status of patients undergoing chronic hemodialysis may influence the prognosis of SARS-CoV-2 infection.