BACKGROUND
In the last years, the provision of clinical nutrition has focused on higher protein intake rather than higher energy intake, especially in severely ill (1) and surgical patients (2), but there is much controversy regarding protein and caloric needs. Current ESPEN guidelines for critically-ill patients recommend 1.3 g protein/kg/day and 20- 25 kcal/kg/day (3). For surgical patients, there are currently no specific intake recommendations (4), but in the previous 2017 guidelines, requirements were estimated at 1.5 g protein/kg/day and 25-30 kcal/kg/day (5). ASPEN guidelines differ from these European guidelines, being 1.2-2 g protein/kg/day and 12-25 kcal/kg/day for critically ill patients (6) and without recent guidelines for surgical patients. In other recommendations, a moderately hypocaloric nutrition, 20 kcal/kg/day, along with a high protein content of 2 g/kg/day, was also proposed for hospitalized patients (7). However, high protein doses and hypocaloric nutrition have not been demonstrated to improve outcomes in critically ill patients (8,9). Most nutritional studies did not examine the relationship between energy and protein intakes (10). An additional variable combining both values could be a logical approach to assess it. This is the classic non-protein calorie to nitrogen ratio (NPCNR) (11), which was early proposed to maximise protein synthesis during clinical nutrition (11). It is worth noting that this is a counterintuitive parameter, the higher the values, the less protein is contained in the diet.
When NPCNR was calculated, the values ranged from 71 to 100 non-protein kcal/g nitrogen for the ESPEN guidelines, from 37.5 to 105 non-protein kcal/g nitrogen for ASPEN guidelines, and about 37.5 non-protein kcal/g nitrogen for hospitalized patients. As can be seen, the proposed nutritional intakes cover a wide range of values. The higher values double and even triple the lower ones. However, to the best of our knowledge, there are no recent studies assessing the influence of NPCNR on nutritional parameters and outcomes in patients receiving parenteral nutrition (PN).
This study aimed to assess the effects of two regimens of isocaloric PN with different NPCNR on the evolution of nutritional parameters and outcomes in adult inpatients requiring this nutritional therapy and receiving intakes close to those recommended.
MATERIALS AND METHODS
STUDY DESIGN
This was a retrospective quasi-experimental study based on prospectively collected data performed in a 400-bed university tertiary hospital in an urban area. All adult (≥ 18 years old) patients were initially eligible if they had received ≥ 4 days of PN, as initial exclusive nutritional therapy, with NPCN ≥ 100 or ≤ 90 between January 2015 and December 2017 during their hospital admission. Patients were excluded if they received long-term (> 90 days) or home PN or lacked recorded data.
Patients were then divided into two initial cohorts depending on their NPCNR: one that included patients who had received PN with an NPCNR ≥ 100 non-protein kilocalories/g of nitrogen and another that included patients who had received PN with an NPCNR ≤ 90 non-protein kilocalories/g of nitrogen. Subsequently, both initial cohorts were propensity-score matched to adjust for differences, resulting in two final cohorts: Cohort “Medium-P” included patients on PN with an NPCNR ≥ 100 who received less protein, and cohort “High-P”, patients on PN with an NCPCNR ≤ 90 who received more protein.
ETHICAL APPROVAL
Ethics approval was obtained from the Comitè Etic d’Investigació, CEIC-Parc de Salut Mar (approval number 2021-9677).
PARENTERAL NUTRITION THERAPY
Overall, PN was designed to provide about 25 kcal/kg ideal body weight (IBW)/day and about 1.2-1.5 g protein/kg IBW/day. The composition of each PN was individually modified when necessary according to clinical conditions, evolution, and laboratory parameters.
PN was prepared following usual hospital practices as an “all-in-one” admixture and was administered in a 24-h perfusion. All patients received the same products used to prepare PN: glucose solutions, standard amino acid solution (Aminoplasmal L, B. Braun, Rubí, Spain), intravenous lipid emulsions (IVLE) (SMOFLipid 20 %, Fresenius Kabi, Barcelona, Spain or Clinoleic 20 %; Baxter; Ribarroja del Túria, Spain), vitamins (Cernevit, Baxter, Ribarroja del Túria, Spain), trace-element solution (Addamel, Fresenius Kabi, Barcelona, Spain or Supliven, Fresenius Kabi, Barcelona, Spain), and electrolytes.
The intravenous lipid emulsions (IVLE) containing fish oil (SMOFLipid 20 %) was used mainly in severely-ill patients or with moderate hypertriglyceridemia (triglyceridemia > 250-400 mg/dL). IVLE based on an olive oil emulsion (Clinoleic 20 %) was used in the remaining patients.
Additional energy sources such as propofol and glucose infusions were also considered.
DATA COLLECTION
Data collected at PN initiation were patient demographics, main diagnosis, anthropometric data (weight, height, body mass index (BMI), IBW (12), type of admission (emergent or elective), type of patient (medical or surgical), critically ill condition, the severity of illness at the beginning of PN, comorbidity, and nutritional risk. The severity was classified as minor (predicted mortality < 10 %), moderate (predicted mortality from 10 % to < 25 %), and major (predicted mortality ≥ 25 %) according to Mortality Probability Model-III (13) at PN initiation. Comorbidity was classified as mild (predicted mortality < 10 %), moderate (predicted mortality from 10 % to < 25 %), and severe (predicted mortality ≥ 25 %) according to the Elixhauser score (14). The nutritional risk was classified as low (score ≤ 1), moderate (score = 2), and high (score ≥ 3) according to the Nutritional Risk Score (NRS) 2002 (15).
Serum levels of biochemical parameters at the beginning and end of PN were also recorded: creatinine and estimated glomerular filtration rate (eGFR) by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (16), albumin, prealbumin, lymphocyte count, C-reactive protein (CRP), triglycerides, bilirubin, and alkaline phosphatase (ALP). Other parameters recorded were the number of days with hyperglycaemia (days with at least one glycemia > 180 mg/dL).
Nutritional data recorded were the mean amount of protein, glucose, IVLE, and energy administered per kg of IBW during PN, mean NPCNR, use of IVLE with fish oil, indication for PN, length of PN, and days between admission and PN initiation.
VARIABLES
Nutritional parameters considered were plasma albumin, prealbumin, cholesterol, and lymphocyte count. The number of patients who improved in at least three of these parameters at the end of the PN was also considered.
Outcomes considered were days requiring PN, admission in intensive care unit (ICU) or post-anaesthesia care unit (PACU) for ≥ 3 days during PN, length of stay (LOS), and mortality 90 days after the end of PN.
All patients were followed for at least 90 days after the end of PN or until death if it occurred before 90 days. Mortality was extracted from hospital records, primary-care records and a central register of the autonomous health authority.
STATISTICAL ANALYSIS
Propensity-score matching was performed to reduce biases in patient selection from the initial cohorts. Propensity scores were obtained by logistic regression and using one-to-one nearest neighbour matching without replacement with assignation to a Medium-P or High-P as a dichotomous dependent variable and sex, age, severity, comorbidity, BMI, plasma albumin, ICU admission, need for renal replacement therapy, all at PN initiation, as independent variables. The caliper was set to a width of twice the standard deviation of the propensity score logit value (17).
Categorical variables were presented as percentages, and continuous variables as mean and 95 % confidence intervals (95 %CI). Analyses were conducted using a chi-squared or Fisher’s exact test for categorical variables and the Student’s t-test for continuous variables. Survival was estimated with the Kaplan-Meier method, and the survival rate was compared using the Breslow test.
In all analyses, p-values were two-tailed and p < 0.05 was considered statistically significant.
Statistical analyses were performed using IBM SPSS Statistics 25.0 (IBM Co., Armonk, NY, U.S.A.).
RESULTS
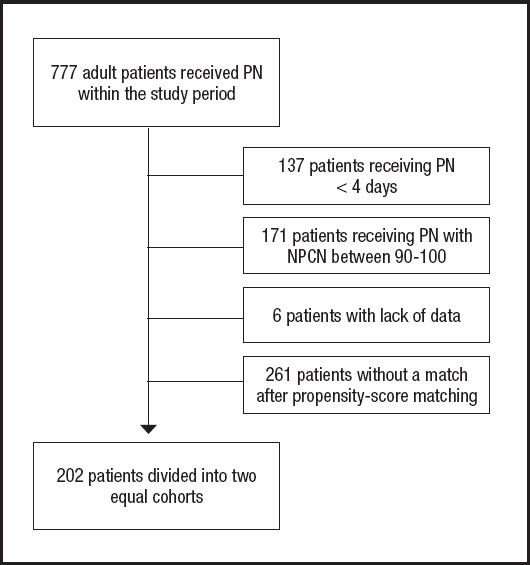
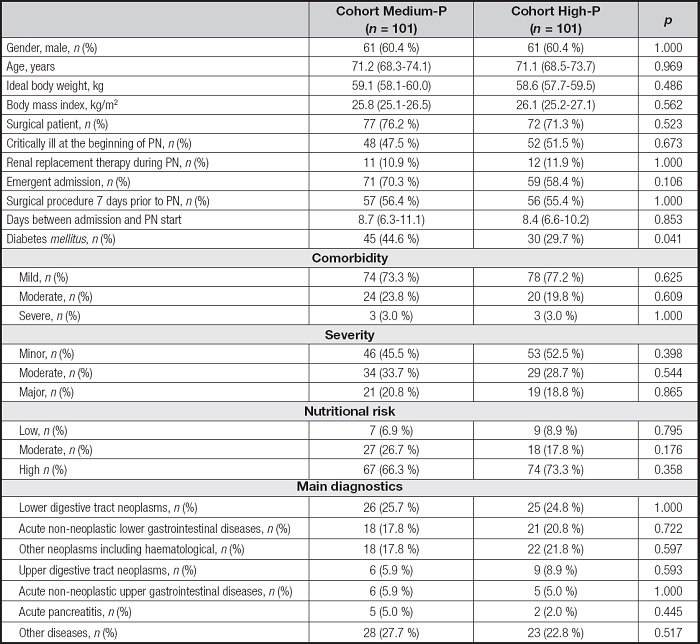
All adult patients receiving PN during the study period were initially screened (Fig. 1). A total of 202 patients were finally recruited and divided into two equal cohorts of 101 patients each. Generally, patients were mainly male (122, 60.4 %), surgical (149; 73.8 %), about half of them critically ill at the beginning of PN (100; 49.5 %), with high nutritional risk (141, 69.8 %) and with neoplasm as the main diagnosis (145; 71.8 %). Detailed baseline characteristics of the two cohorts were presented in table I. The only difference between the two cohorts was the prevalence of diabetes mellitus, being about 15 % higher in the Medium-P cohort.
Baseline biochemistry at the start of PN was presented in table II. The parameters studied did not differ between the two cohorts. PN provided about 25 kcal/kg IBW/day in all patients, but there were differences in protein intake, being about 0.25 g/kg IBW/day higher in the High-P cohort. The dose of lipid emulsion provided was lower in the High-P cohort and NPCNR was lower in the High-P cohort. More patients received IVLE-containing fish oil in the High-P cohort.
Table II. Baseline biochemistry and nutrition related parameters.
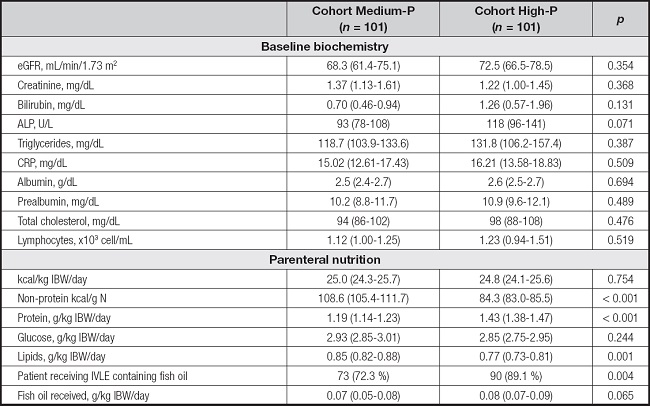
ALP: alkaline phosphatase; eGFR: estimated glomerular filtration rate; CRP: C-reactive protein; IBW: ideal body weight.
Few differences were detected in the evolution of biochemical and nutritional parameters to the end of PN. Data were shown in table III. The difference in triglycerides and lymphocytes was lower in the High-P cohort. This cohort also presented more days with hyperglycaemia during PN.
Table III. Evolution of biochemical and nutritional parameters at the end of PN.
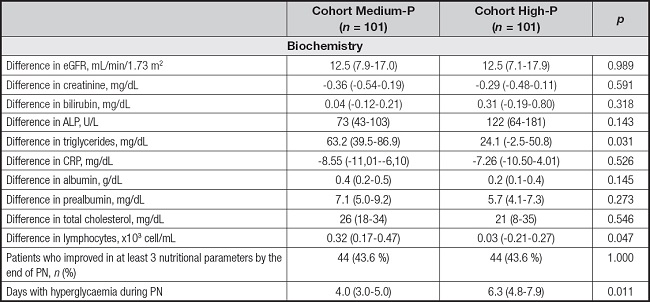
Evolution, difference between values at the end of PN in relation the baseline values at PN initiation. ALP: alkaline phosphatase; eGFR: estimated glomerular filtration rate; CRP: C-reactive protein.
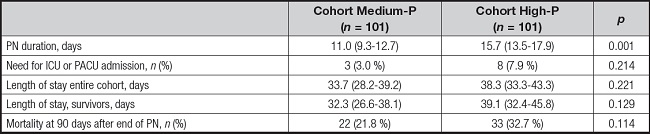
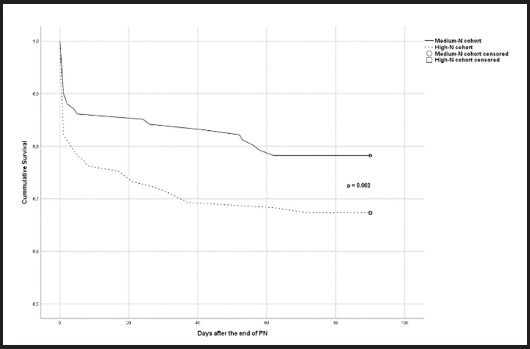
Outcomes were not statistically different between the two cohorts, except for PN duration, which was longer in the High-P cohort (Table IV). Mortality reached a p value close to significance when analysed by a Kaplan-Meyer plot, as shown in figure 2.
The sub-study in critically ill patients included a total of 100 pa- tients distributed as shown in table I. Baseline characteristics of both subgroups were similar without statistical differences except for the protein intake, sub-cohort Medium-P received 1.19 (1.13-1.25) vs. 1.45 (1.40-1.51) g/kg IBW/day received by sub-cohort High-P, p < 0.001, and, evidently, for NPCNR 108.5 (104.2-112.9) vs. 84.8 (83.2-86.5), respectively, p < 0.001.
The results only differed in days requiring NPT, sub-cohort Medium-P 10.2 (8.4-12.0) vs. 16.9 (13.2-20.6) days in sub-cohort High-P, p = 0.002. Mortality rate was not different.
The sub-study in patients receiving fish oil in the IVLE included 163 patients distributed as shown in table II. Baseline characteristics of both subgroups were similar without statistical differences except for the protein intake 1.21 (1.17-1.25) vs. 1.42 (1.38-1.47) g/kg IBW/day, p > 0.001; non-protein kcal/g N 20.1 (19.5-20.7) vs. 19.1 (18.5-19.7) NP kcal/kg IBW/day, p = 0.031; and NPCNR 103.9 (102.2-105.7) vs. 84.4 (83.1-85.8), p < 0.001. Both sub-cohorts received the same fish oil intake, about 0.09 (0.08-0.10) g/kg IBW/day. Results differed only in difference in prealbumin 9.0 (6.1-12.0) vs. 6.0 (4.4-7.5) mg/dL, p = 0.044; and in days requiring NPT, 10,8 (8.1-13.6) vs. 18.0 (14.3-21.8), p = 0.008. In all cases, being the first values for the sub-cohort Medium-P and the second values for the sub-cohort High-P.
The results in these sub-studies are in consonance with the results obtained in the general study.
DISCUSSION
This study found no advantages in providing moderately high protein doses with lower NPCNR in adult patients receiving an isocaloric PN regimen versus medium protein doses with higher NPCNR, even with several parameters worsening, e.g., days requiring PN, lymphocyte count evolution, or days with hyperglycaemic episodes. Mortality differences approached significance, being higher in the cohort receiving moderately high protein doses. The results of sub-analysis in critically-ill patients or in patients receiving fish oil in the IVLE did not differed significantly from the entire cohort. This study had several differences compared with most studies consulted. It included mixed patients, critically and non-critically ill, studied an isocaloric diet and the relationship between calories and protein delivered, provided protein doses close to those recommended, and it was performed with PN as the exclusive nutritional therapy. All these points, to our knowledge, have not been previously explored.
STUDIES FOCUSED ON PROTEIN PROVISION
Much controversy has been generated on protein provision in clinical nutrition (18) and most analyses have focused only on protein and critically ill patients. Three recent meta-analyses reviewed this topic. That of Fetterplace et al. included six trials with 511 critically ill patients receiving exclusively enteral nutrition (EN) (19). They found no differences in functional outcomes, mortality, and LOS between a high protein group (1.3 g protein/kg/day) and a low protein group (0.75 g/kg/day). The two groups also received different energy provisions (21 kcal/kg/day vs. 17 kcal/kg/day). NPCNR values were around 76 in the high and 116 in the low protein group. Lee et al. (20) included 19 randomized controlled trials (RCT) with a total of 1731 patients, only three of which were exclusively PN. They concluded that 0.48 g/kg/day higher protein delivery (1.31 ± 0.48 vs. 0.90 ± 0.30 g/kg/day) with similar energy provided (around 20 kcal/kg/d) had no significant effect on overall mortality, infectious complications, mechanical ventilation (MV) duration, and length of ICU and LOS. The only difference was muscle loss attenuated in the higher protein group. This difference was mostly attributed to enteral nutrition (EN) studies. When calculated, the NPCNR for the higher protein cohort was about 70, and about 114 for the lower protein cohort. A prior meta-analysis (21) also found no effects on mortality from different protein doses delivered (0.67 vs 1.02 g/kg/day).
In PN, protein doses were compared in the RCT of Ferri et al. (22) who found that providing 1.2 g of protein/kg/day vs 0.8 g/kg/day in a non-isocaloric regimen in critically ill patients improved handgrip strength, fatigue score, and muscle thickness, but with no differences in LOS, ventilator days, and mortality. The NPCNR in this study could be calculated as 98 for the high protein cohort and 148 for the low protein cohort. Additionally, a recent international study including data from 16,489 critically ill patients (23) found that an enteral or parenteral intake of 0.8- 1.2 g protein/kg/day during the late acute phase was associated with lower hospital mortality versus a higher protein intake (> 1.2 g/kg day).
STUDIES FOCUSED ON THE PROVISION OF PROTEIN AND ENERGY SEPARATELY
Few additional studies have analysed both energy and protein delivered in clinical nutrition. These variables are usually studied separately. A metanalysis for protein delivered (24) included 14 RCTs with a total of 1690 patients. The protein doses compared were 1-2 g/kg/d vs. 0.5-0.9 g/kg/day. No changes were detected in daily living activities after discharge, handgrip strength, quality-of-life score, mortality, and LOS. A significant increase in muscle mass was noted with high protein delivery. Regarding energy, the same study meta-analysed 15 RCTs including 3892 critically ill patients. No outcome differences were detected between high (≥ 20 kcal/kg/day) versus low (< 20 kcal/kg/day) energy delivered. NPCNR could not be calculated from the data provided.
The prospective observational multinational EuroPN study (25) assessed nutritional practices in European ICUs, recruiting a total of 1172 critically ill patients. Protein and energy intakes were analysed separately, finding that 10-20 kcal/kg/day was associated with longer survival times and shorter invasive MV times and 0.8-1.2 g protein/kg/day was associated with earlier weaning from MV, but not survival.
STUDIES FOCUSED ON ENERGY PROVISION
Energy delivered and its effects were analysed in a recent review (26), where only six RCTs including 1143 critically ill patients addressing this question were found. It concluded that achieving energy balance with clinical nutrition may improve outcomes, but too many uncertainties remain to make this a strong statement.
STUDIES FOCUSED ON THE RELATIONSHIP BETWEEN PROTEIN AND ENERGY
This relationship has been much less explored. From early studies, a NPCNR of 100-150 was proposed to permit anabolism during the anabolic phases of convalescence (11). In a much more recent review, Kreymann et al. (10) found a non-linear relationship between total protein loss and the energy/nitrogen ratio provided. The study included 91 cohorts of patients and healthy subjects. They inferred a single equation for all cohorts. Hospitalized patients had a lower energy/nitrogen ratio than healthy subjects. Moderately ill patients had a mean calculated NPCNR of 166 and severely ill patients, 96. However, the variable energy/nitrogen ratio did not provide additional information to NPCNR as nitrogen is in the numerator, as calories provided by proteins, and also in the denominator as nitrogen itself. This ratio can be easily converted to NPCNR by just subtracting 25.
STUDIES FOCUSED ON VARIABLES AFFECTING MORTALITY
In two different studies, the effects of nutrition therapy on mortality, amongst other variables, were evaluated. In critically ill patients requiring artificial nutrition (PN, EN, or mixed), Servia et al. (27) analyzed 639 patients finding that providing more protein and fewer calories was protective for 28-day mortality. However, they provided only a mean of 16 kcal/kg/day and 0.8 g of protein/kg/day. In a similar study, Mateu and Retamero (28) analyzed 634 mixed patients exclusively receiving PN, finding that the provision of more energy was protective for 90-day mortality. In this study, patients received a mean of 25 kcal/kg IBW/day. Protein delivery did not affect mortality.
Taken together, our results seemed to agree with the conclusions from all these studies. The provision of higher protein doses has no notable advantages compared to most “conventional” doses.
This study had several limitations. First, its retrospective nature, although a propensity-score matching was performed to reduce biases. This is a single-center study. The results could not be extrapolated to special populations such as patients with obesity or low BMI, with high risk of refeeding syndrome, burns, under extracorporeal membrane oxygenation, or severe polytrauma. The extrapolation to EN could also be difficult since PN could negatively modify gut hormones, bile acids (29), microbiota (30), and metabolism (31). Variation parameters for muscle mass or performance were not been included in the study as they are not routine practices in clinical settings. Time of PN initiation (early versus late) was also not studied, although days from admission to PN initiation were similar between cohorts. Neither were the different energy and protein doses in the early phase of PN studied. This study had a quasi-experimental design and a further RCT would confirm the results.
In conclusion, in adult patients requiring PN, the provision of energy close to that recommended with moderately high protein doses and lower NPCNR did not present any advantages and even worsened some secondary parameters compared to providing the same energy with medium doses of protein with higher NPCNR. Differences in mortality approached significance, being higher in the cohort receiving moderately high doses of protein.