INTRODUCTION
Obesity has become a significant global health problem due to its strong association with diseases such as insulin resistance, type 2 diabetes, atherosclerosis, and ischemic heart disease, which reduce life expectancy and have significant economic and societal impacts. Obesity is associated with inflammatory cytokine (IC) release in white adipose tissue (AT), triggering local inflammation, as well as with low-grade chronic inflammation in adipocytes (1,2). Furthermore, the pathogenesis of obesity-related metabolic disorders is known to play a crucial role in chronic inflammation. Low-grade inflammation has been clearly linked to metabolic disorders such as type 2 diabetes mellitus (DM). Increased AT mass leads to elevated pro-inflammatory markers such as C-reactive protein (CRP) and its inducer, interleukin (IL)-6. Interestingly, elevations in CRP and IL-6 were found to predict the development of type 2 DM (3,4). AT also causes the production of inflammatory mediators known as adipokines; when released into the circulation, these transport the local inflammation caused by obesity to other parts of the body, including cardiovascular tissues (5).
In obesity and diabetes, tumor necrosis factor-alpha (TNF-α) has been identified as a pro-inflammatory product of AT which links obesity and inflammation (6). A previous study revealed that mice with inactivated insulin receptors are protected from obesity-related insulin resistance and inflammation (1). This shows that in obesity, the insulin receptor plays a key role in macrophage activation and inflammation (2). Accordingly, obese children also had increased circulating levels of pro-inflammatory cytokines (e.g., leptin, TNF-α, IL-6, and CRP) compared to normal-weight children and adolescents aged 2-18 years (3). Specifically, TNF-α activates macrophages in host defense mechanisms, inducing the production of pro-inflammatory nitric oxide and reactive oxygen species (7).
IL-10 has been recognized as an anti-inflammatory and immunosuppressive factor. As an anti-IC, IL-10 suppresses the ability of human monocytes and macrophages to produce pro-ICs, including IL-6 (8). IL-10 is produced by activated immune cells (T and B lymphocytes, mast cells, granulocytes, macrophages) which acts as a potent negative feedback regulator and controls inflammation (7). Within lean AT, anti-ICs secreted by resident AT macrophages (ATMs) help maintain insulin sensitivity by counteracting inflammatory responses. Indeed, treatment of adipocytes with IL-10 alleviated the insulin resistance (IR) induced by TNF-α (9).
To the best of our knowledge, no study has evaluated the role of IL-10 in childhood obesity. This study aimed to identify pro-inflammatory and anti-inflammatory markers present in children and adolescents to determine their relationship with obesity.
MATERIALS AND METHODS
Children and adolescents (40 obese, 46 controls) were recruited from the pediatric outpatient department of the hospital of Tokat Gaziosmanpasa University School of Medicine, Tokat, Turkey. The study was conducted in accordance with the Helsinki Declaration of the World Medical Association and local ethical standards; this was approved by the Ethics Committee of Gaziosmanpasa University School of Medicine (approval no. 17-KAEK-014). Since can cause inflammation, subjects with symptoms or signs of infection, fever, or/and endocrinologic disorders such as thyroiditis or rheumatologic diseases such as arthritis or rash were excluded. The study group was classified according to the age and sex-adjusted body mass index (BMI) reference curve for Turkish children. Participants with BMI above the 95th percentile were classified as obese, while those between the 5th and 84th percentiles as normal (10) (Table I).
Serum samples were obtained from fasting blood samples and stored at -80 °C. TNF-α and IL-10 were measured using an ELISA kit (Elabscience Biotechnology Co., Wuhan, China). Laboratory tests, including complete blood count, glucose, blood urea nitrogen, serum creatinine (sCre), CRP, aspartate transaminase (AST), and alanine transaminase (ALT), were obtained from fasting blood samples of all participants. Insulin levels and thyroid function tests were measured in obese participants only. Serum fasting glucose, insulin, AST, ALT, and sCre were measured using reagent kits from Roche Diagnostics adapted to the COBAS E601 system (Roche Diagnostics, Mannheim, Germany). Statistical analysis of the data was performed using SPSS 19 (IBM SPSS Statistics 19, SPSS Inc., Somers, NY, USA), and p < 0.05 was considered significant.
RESULTS
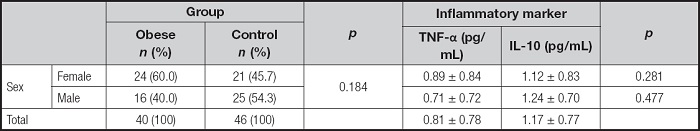
Serum TNF-α and IL-10 levels were measured in 40 obese children and 46 controls, who had mean ages of 12.7 ± 3.2 and 13.1 ± 2.3 years, respectively (p = 0.698). Among the participants, 53.5 % were female. When grouped according to sex, there were no significant differences in TNF-α and IL-10 levels. TNF-α levels were 0.892 ± 0.835 and 0.708 ± 0.716 pg/mL in females and males, respectively (p = 0.281). IL-10 levels were 1.117 ± 0.832 and 1.238 ± 0.705 pg/mL in females and males, respectively (p = 0.477) (Table II).
Table II. Distribution of TNF-α and IL-10 serum levels and laboratory parameters by groups.
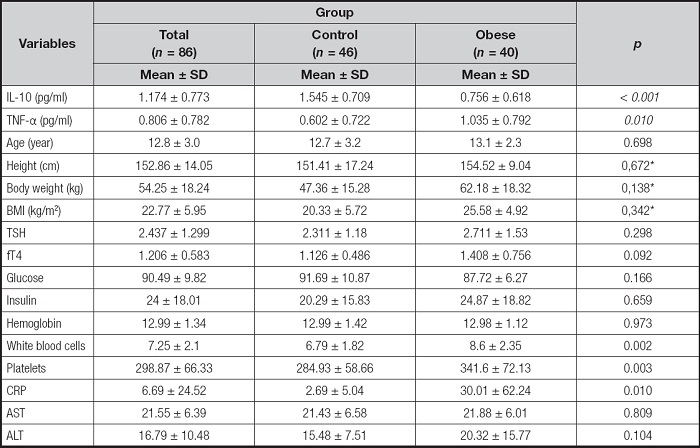
TNF-α: tumor necrosis factor-alpha; IL-10: interleukin-10; BMI: body mass index; TSH: thyroid stimulating hormone; fT4: serum free thyroxin; CRP: C-reactive protein; AST: aspartate transaminase; ALT: alanine transaminase. The values presented are means ± standard deviation (SD). The p-values represent the statistical significance for the comparison between the control and obese groups.
*Correction for age and gender was performed by covariance analysis.
Serum TNF-α levels were significantly higher in obese individuals versus controls (1.04 ± 0.79 vs. 0.60 ± 0.72 pg/mL, p = 0.010) IL-10, known for its anti-inflammatory properties, was lower in obese individuals versus controls (0.76 ± 0.62 vs. 1.54 ± 0.71 pg/mL, p < 0.001).
DISCUSSION
Chronic inflammation, as seen in obesity, can occur due to excess production of inflammatory mediators when clearance mechanisms are insufficient or even under normal physiological changes. Circulating inflammatory mediators and activated monocytes have been associated with metabolic and cardiovascular complications in obesity (5). In this study, we aimed to assess inflammation by determining the levels of inflammatory markers TNF-α and IL-10. Notably, TNF-α, which triggers inflammation by promoting macrophage activation and oxygen radical production (8), was higher in obese children and adolescents. IL-10, which has anti-IC actions and suppresses monocyte/macrophage stimulation and cytokine production, especially IL-6 (7), was lower in obese children and adolescents.
Previous studies have associated subclinical chronic inflammation with obesity (11). AT acts not only as a fat depot but also as an active endocrine organ which releases numerous peptides and cytokines into the circulation (12). In obesity, there is a shift in the balance among these molecules; enlarged adipocytes produce more pro-ICs (e.g., TNF-α and IL-6) and fewer anti-inflammatory peptides (e.g., adiponectin) (13). The infiltration of macrophages into AT was also found to increase with the severity of obesity, which is associated with decreased insulin sensitivity (14). The dysregulation of these adipokines reportedly plays a significant role in obesity-related metabolic and cardiovascular disorders (15). Severely obese adolescents with increased BMI and CRP have an increased risk of impaired fasting glucose and hypertension (16). Similarly, regardless of the association with metabolic complications, we also found elevated TNF-α levels in obese children and adolescents. Kopp et al. reported that TNF-α levels decreased alongside weight loss in obese patients with metabolic syndrome (17). Thus, weight loss can possibly achieve inflammation control in obesity-related metabolic disorders.
Protective factors, such as adiponectin, reportedly decrease in cases of increased BMI and elevated CRP (18). IL-10 is considered a good and protective cytokine in human metabolism (19). In atherosclerosis, IL-10 is speculated to be produced in the atherosclerotic plaques by stimulated monocytes/macrophages and lymphocytes, and it may provide more protection from excessive pro-inflammatory responses (20). Jung et al. showed that an increase in IL-10 was dependent on the degree of weight loss and improvement in metabolic disorders (21). In our study, IL-10 levels were low in obese adolescents and children. Since we did not observe the effects of weight loss, we cannot make interpretations about the changes in this cytokine. In contrast, it has been suggested that IL-10 release is related to TNF-α and could help limit the pro-inflammatory effect of TNF-α (21).
These studies have shown that low-grade systemic inflammation is present in childhood obesity. Consequently, it has been proposed that metabolic dysfunction associated with excessive AT mass could result from an imbalance in the expression of pro- and anti-inflammatory adipokines, thereby contributing to the development of obesity-related complications (5,22).
A limitation of this study is the lack of investigation into the relationship between metabolic disorders and inflammatory markers. Nevertheless, as a case-control study, the clear differences in these markers highlight the importance of our findings.
In conclusion, this study examined two inflammatory markers in childhood obesity, TNF-α and IL-10. Inflammatory mediators differed between obese and lean control groups. These ICs may be part of the inflammatory connection between obesity and its metabolic and cardiovascular complications. Thus, these can be potential targets for the early detection and prevention of these complications.