Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
The European Journal of Psychiatry
versión impresa ISSN 0213-6163
Eur. J. Psychiat. vol.24 no.2 Zaragoza abr./jun. 2010
The early Kraepelin´s dichotomy of schizophrenia and affective disorder - Evidence of separate diseases?a
H. Häfner
Professor emeritus of Psychiatry, University of Heidelberg. Head, Schizophrenia Research Unit, Central Institute of Mental Health. Mannheim. Germany
The ABC study was funded by the German Research Association (Deutsche Forschungs-gemeinschaft) as part of the Special Research Branch (Sonderforschungsbereich) 258 at the Central Institute of Mental Health until December 1998 and from January 1999 to Sept. 2002 as an independent project.
ABSTRACT
Background and Objectives: Testing Kraepelin´s dichotomy model, we studied the separability of schizophrenia and affective disorders by their symptoms and course.
Methods: To this end symptoms and illness course were assessed retrospectively in individually matched untreated probands with schizophrenia and depression (n=130 each) from first admission back to illness onset in comparison with 130 "healthy" controls. In a second study these same variables were studied prospectively in 107 patients with schizophrenia over a homogenised follow-up of 134 months (11.2 years). The actual mean length of the follow-up period was 12.3 years.
Results: The symptom most frequently marking the onset of both schizophrenia and depression was depressive mood. Both disorders exhibited the same prodromal core syndrome. It was not until the emergence of positive symptoms that the disorders became separable by the international classification systems. Depression remained the most frequent syndrome over the course of schizophrenia.
Conclusions: Obviously, depression does not represent comorbidity, but an integral part of psychosis. A dimensional disease model based on (successively emerging) hierarchical symptom patterns of the human brain with increasing brain dysfunction in the course of schizophrenia and several neuro-degenerative disorders, not unknown to the later Kraepelin, is offered as an explanation.
Key words: Schizophrenia; Affective disorder; Course; Prodomal symptoms; Symptom dimensions.
Introduction
With biological discriminative criteria lacking the early Kraepelin1 defined the two major psychoses, dementia praecox and manic-depressive insanity, by their symptoms and course. Although discarded by Kraepelin2 in his later days, this dichotomy of two discrete disease entities has survived in our diagnostic classification systems and clinical practice. For this reason the issue deserves our attention.
Studying the course of schizophrenia and its main symptom dimensions -psychotic, negative, depressive and manic- over a period extending from illness onset to about 12 years after first admission we will draw some epidemiological comparisons between the two disease constructs.
Kendel & Brockington3 studied the empirical separability of the constructs of schizophrenia and affective disorder in the population at large. They found no "point of rarity", i.e. no decrease in symptom frequencies to chance value between the characteristic syndromes. The question whether modern neurobiological findings might provide indicators for the validity of the dichotomy needs to be addressed at different levels. The apparently disease-specific efficacy of pharmacotherapy is not a proof of the validity of these disease constructs. Ever since Freyhan4 numerous authors have demonstrated that antidepressant and antipsychotic substances act on target symptoms or syndromes independently of the disease constructs involved.
On the genetic level, twin and family studies and studies of the offspring of women with schizophrenia have demonstrated different degrees of familial cosegregation of the risk for bipolar, unipolar, schizoaffective and schizophrenic psychoses5-10. On the molecular level systematic genome-linkage studies, focussed linkage studies and association studies have yielded a limited number of gene loci and haploid genes associated with an increased risk for a disorder of either psychotic or affective type, frequently also for both types6, 11-13.
The most important gene products associated with susceptibility for both schizophrenia and affective disorder are NRG1 (neuregulin 1), on chromosome 814,15, DAOA (diaminooxydase activator) on the G72/G30 gene on chromosome 1316,17 and in rare cases DISC1 (disrupted in schizophrenia 1)18. Craddock & Owen19 stress that these genes straddle the Kraepelinian divide car rying risk for both types of disorder. A plausible explanation could be offered by a polygenetic model, as in the case of the risk for coronary heart disease, associated both with high blood pressure and increased blood fat levels. According to such a model, underlying the symptom patterns in question are several neurophysiological dysfunctions, in the causation of which several genes may play a role20.
A psychological risk factor demonstrated by van Os et al.21 is the neuroticism personality trait. It is associated with a risk for both syndromes. Environmental factors are more difficult to prove, as they may contribute to the manifestation of illness by interacting with the genetic predisposition. Growing up in an urban environment22, migration23 and being member of an ethnic minority group24 have been confirmed as risk factors. But here it is difficult to distinguish between social selection and social causation, because the genetically determined neural dysfunctions increase not only the risk for psychosis, but also the probability of more or less deviant personality traits, which, on their part, affect social bonding and expansive behaviour -"novelty seeking"-, thus influencing selection forces in migration processes. The pioneer of psychiatric epidemiology, Ørnulf Ødegaard25, early demonstrated the role such factors may play in the migration of individuals at risk.
To conclude thus far: not a single powerful and reliable criterion has been found yet that clearly discriminates between the two Kraepelinean disease entities.
The course-related criterion the early Kraepelin used for distinguishing between the two disease constructs held that manic-depressive illness usually runs a remitting type of course without affecting the patients´ intelligence, whereas dementia praecox is characterised by a therapy-resistant mental defect growing more and more severe with each "florid" episode of psychosis and finally resulting in dementia. It was this trajectory of a descending staircase that the authors of the NIMH follow-up study26, too, proposed, although their empirical data did not justify it.
Differences in the social course of the two disorders have been studied repeatedly and used as a criterion for their discreteness. Tsuang et al.27 in their Iowa-500 Study showed that schizophrenia has the most unfavourable, bipolar affective disorder the most favourable course -apart from surgical controls- with schizoaffective psychoses occupying an intermediate position. A more recent study conducted by Marneros et al.28, too, came to a similar conclusion on the basis of proportions of patients in independent versus dependent living situations. But this frequently confirmed finding, too, fails to provide a reliable criterion for distinguishing between the two disorders, because their social courses tend to reflect differences in the severity of illness in these diagnostic groups and are subject to interaction with the environment. The high degree of variation in the illness courses of the two syndromes does not make matters easier either.
If we proceed from operational definitions of the diagnoses adjusted for severity of illness29 the long-term courses of the disorders are even harder to distinguish. Testing the stability of six diagnostic clusters, the long-term follow-up study of Marneros et al.28, covering a mean follow-up period of 23 years (range: 10 to 50 yrs.) following admission to hospital and based on diagnoses not adjusted for severity of illness, found that "uniform", chronic syndromes such as schizophrenia and major depression -which the authors called melancholy- were comparatively stable. In contrast, "multiple" syndromes, such as bipolar and schizoaffective disorders, showed a high degree of instability. The problem we are faced with here is that the probability of change reflects the number of syndromes and their degree of chronicity in the long-term course. To conclude, attempts to validate Kraepelin´s dichotomy by illness course-related criteria have also failed.
Own studies
For this reason, we set out to compare schizophrenia and depression after illness onset and to analyse the long-term course of the affective and non-affective symptom dimensions of schizophrenia.
Material and methods
As described in our first publications from the ABC (Age, Beginning, Course) Schizophrenia Study30,31, we collected data on all first admissions for schizophrenia spectrum disorder (ICD -International Classification of Diseases -9: 295, 297, 298.3, 298.4) in age range 12 to 59 years in a two-year period from a semi-rural, semi-urban population of 1.5 million (Heidelberg, Mannheim, Ludwigshafen, Rhine-Neckar District, eastern Palatinate). Of the 276 patients interviewed 232 were in their first psychotic episodes. To distinguish prodromal signs from symptoms generally occurring in the population, we also assessed 130 "healthy" controls from the population of origin, matched by age and sex with a representative subsample of 130 first admissions for schizophrenia and 130 equally matched first admissions for moderately severe (F32.10, 32.11) and severe depression (F32.2, 32.30, 32.31). Immediately on hospital admission the patients went through interviews by the PSE - Present State Examination -32, SANS - Scale for the Assessment of Negative Symptoms -33, PIRS - Psychiatric Impairments Rating Schedule -34 and DAS - Psychiatric Disability Assessment Schedule -35,36. Two to 4 weeks later, after acute symptoms had remitted, they were administered an IRAOS - Interview for the Retrospective Assessment of Schizophrenia - interview37-39. In the controlled study of illness course we assessed 115 first illness episodes (from among the 130 first admissions) retrospectively back to illness onset and followed up this subsample prospectively at six cross-sections over five years and at a 7th after a mean of 12.3 years after first admission. The healthy controls were assessed using the same instruments as with the probands. At the final follow-up matched controls from the population of the study area were interviewed on the phone using shortened versions of the instruments in order to obtain standard values for symptom variables and social parameters, for example on changes in the unemployment rate of the population.
At five-year follow-up 103 of the 130 first-admission patients and at 12.3-year follow-up 107 patients from the initial first-episode sample of 232 patients could be reached and interviewed. The patients´ mean age at the final follow-up was 42 years (range: 29 to 67). 24 patients (10.3%) had died, 15 (= 6.5%) of them of suicide. The follow-up sample was representative of the initial sample, which we tested on demographic and some illness-related data, but found no significant differences40.
Results
Early course
Of the 115 patients diagnosed as suffering from schizophrenia 80% and of the 115 patients diagnosed at first admission as suffering from severe or moderately severe depression 79% had not previously received any antidepressant or antipsychotic medication41. Hence, we were able to study symptomatology more or less unaffected by specially targeted drugs.
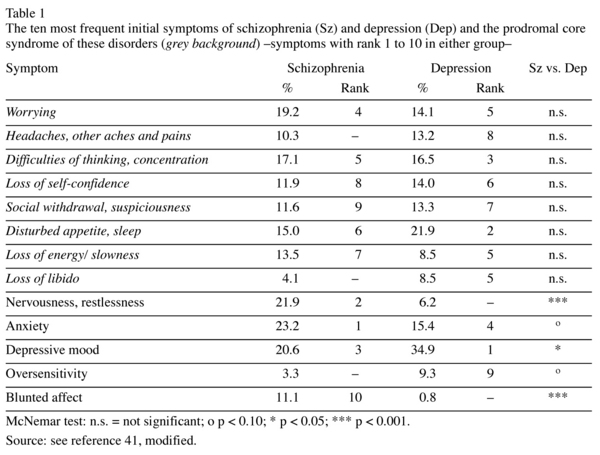
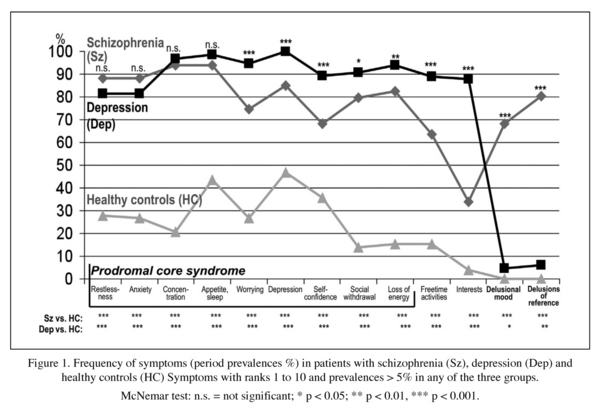
Table 1 gives the initial symptoms most frequent in the two illness groups, a total of 13. Eight of these 13 symptoms had almost equal frequencies and similar rankings in both the schizophrenia and the depression group. As expected, the period prevalences of the 10 most frequent symptoms, assessed over the entire early illness course, -these symptoms reflect the main prodromal symptomatology of schizophrenia and depression- were significantly higher in the two illness groups, schizophrenia and depression, than for healthy controls. (Figure 1). The most frequent prodromal symptoms -restlessness, anxiety, difficulties of concentration, disturbed appetite- showed no significant differences in frequency between the two groups. The remaining symptoms, except the psychotic symptoms depicted on the right-hand side in the Figure, while significantly differing in frequency, have prevalences fairly similar in size and ranking, e.g. depressed mood in the depression group 100% (rank: 1), in the schizophrenia group 84.9% (rank: 5), worrying: 94.6% (rank: 4) versus 74.6% (rank: 9), loss of energy/slowness 93.8% (rank: 5) versus 82.5% (rank: 6), social withdrawal /suspiciousness 90.8% (rank: 6) versus 79.8% (rank: 8), lack of self-confidence 89.2% (rank: 7) versus 68.3% (rank: 10), reduced free-time activities 89.1% (rank: 8) versus 63.5% (-). The symptoms included in the parenthesis are the ones that were already counted among the initial symptoms. That illustrates not only the high degree of similarity between the prodromal symptoms of the two disorders, but also the stability of these symptoms in the early illness course.
The non-psychotic symptom profiles of schizophrenia and depression before the onset of psychotic symptoms seem to be almost identical. It is the two delusional symptoms, which show high prevalence rates in schizophrenia, but in depression very low rates hardly different from those for healthy controls, that represent a criterion for discriminating between the two disorders. Since their onset does not occur until late at the prodromal stage, usually in the last year preceding the climax of the first psychotic episode, the non-psychotic prodromal stages in the two disorders remain inseparable until then.
But the apparently reliable discrimination between the two diagnostic groups on the basis of positive symptoms is an artefact, because in view of the hypotheses we intended to test we had selected the probands on the basis of their diagnosis. In the course of our retrospective analyses we landed back at the inseparable prepsychotic prodromal stage of the two diagnoses. After that stage the psychotic symptoms at first admission were also included in the comparisons, and, in accordance with the diagnostic definitions of the two groups, a clear-cut distinction between them emerged.
Long-term course
Outcome analysis
A comparison of patients at first admission and at 12.3-year follow-up showed an impressive decrease in the proportion of those presenting symptoms and social disability (Table 2). But this result, too, was an artefact. At first admission practically all patients were in a psychotic episode exhibiting maximum symptom scores. At follow-up we had a random selection of patients in episodes and intervals with a mean of 22% in psychotic episodes.
A comparison of marriage and partnership showed that the proportion of patients living alone had decreased considerably and the proportion of married, especially among women with schizophrenia, had increased markedly, but so had also the proportion of divorced patients (Table 3). Compared with healthy controls, however, patients suffered from pronounced social disability. A male-female comparison showed that, despite their illness, clearly more women than men were able to find a partner and tie the knot (Table 4). But the high rate of divorce also indicated that a lot of these marriages did not last for long. The high rate of single male patients is reflected in the above-average proportion of men among psychiatric home residents.
These sex differences are accounted for by normal sex differences in social behaviour rather than by the biological illness, which affects men and women in the same way.
Analysis of illness course
To prevent the high degree of variation in the durations of illness and its course from distorting the results we based our analyses instead of the mean follow-up period of 12.3 years on the shortest follow-up period of 11.2 years or 134 months. The uniform duration of illness and monthly steps of analysis allowed us to closely monitor the presence of symptoms and changes in social and other parameters.
During the 134-month period following first admission we counted a total of 333 psychotic relapses -defined by deterioration in psychotic symptoms of at least 14 days´ duration, preceded and followed by four weeks in which symptoms were absent or reduced- a mean of three and a range of 0 to 29 per patient. The psychotic episodes usually contained a certain proportion of depressive symptoms. 73 (range 0 to 11/patient) or 18% of the relapses were mainly depressive in type with no psychotic symptoms occurring.
We studied the course of clinical symptom categories on the basis of five traditional syndromes. The months patients spent with depressive symptoms clearly predominated. Months spent with negative, positive and manic symptoms were clearly rarer (Table 5).
To avoid overlap between the symptom categories we introduced discrete core syndromes in our analyses. In each group we selected the most frequent non-overlapping symptoms. The depressive core syndrome consisted of four symptoms different from negative symptoms, the manic syndrome consisted of five symptoms separate from psychotic symptoms. To define a psychotic core syndrome -a difficult task given the great number of such symptoms- we selected the four most frequent ones from among Kurt Schneider´s symptoms of first rank (cf. Table 6) excluding those with prevalences below 1%b. Since in the IRAOS interview the complex negative syndrome comprises a total of 19 partly heterogeneous single symptoms, we refrained from defining a negative core syndrome.
Again, a depressive symptom, depressed mood, turned out to be the predominant symptom of schizophrenia (Table 6). A comparison of episodes and intervals showed that the depressive core syndrome was present in 44% (median: 36%, range 0-100) of psychotic episodes and in 34.5% (median: 6%; range 0-100)) of intervals. The mean-median difference indicates a skewed distribution.
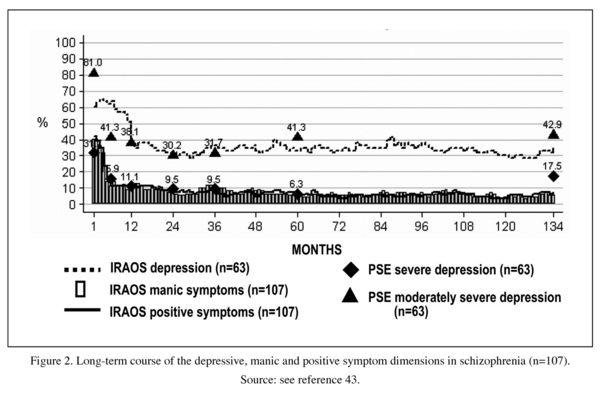
The montly prevalences of the three core syndromes over the entire follow-up period of 134 months are illustrated in Figure 2. The retrospectively assessed prevalences for depressive symptoms were validated on prospective data gathered at seven cross-sections. The black triangles stand for PSE-CATEGO-based frequencies of severe and moderately severe depression, the black quadrangles for those of severe depression. The prevalences of all the five syndromes declined more or less steeply after the first episode. In the subsequent course they all showed a plateau. Depression remained the predominant syndrome throughout the follow-up period. There was visible neither a trend of increase or decrease in symptoms nor a staircase-like deteriorating course, as postulated by Kraepelin.
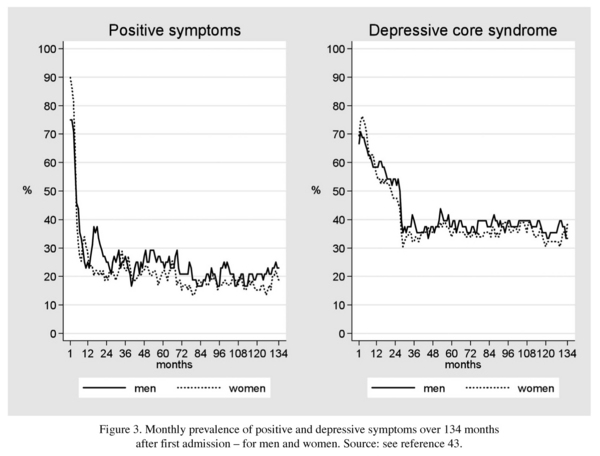
A comparison between males and females showed no difference in the frequency and course of psychotic and depressive symptoms (Figure 3). This finding supported our conclusion that unlike age at onset or the medium-term social course45, the disorder as such is the same in men and women.
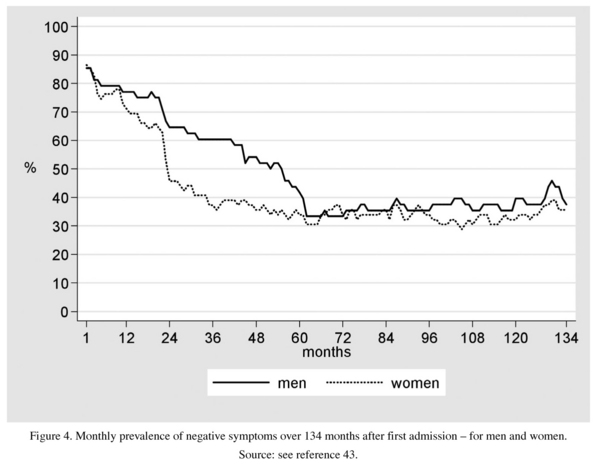
Negative symptoms, assessed on the basis of raw, not necessarily non-overlapping data, showed a different course (Figure 4): a slow, pronounced initial decrease followed by a stable plateau after about five years, as was the case with the other symptom dimensions, too. The male prevalences were slower to decline than the female ones. Accounting for this sex difference, too, might be not the disorder as such, but typically male and female behaviours.
Behind these mean values, which convey the impression of a homogeneous illness course, there lies a high degree of interindividual variability. 19% of the cases experienced only one single episode and no relapse episodes, symptoms or signs of disability. But there were also extremely poor courses involving cognitive impairment and considerable functional disability.
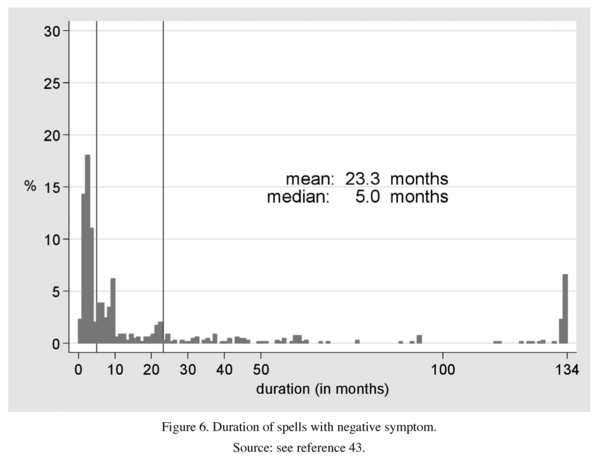
We also tried to assess the individual time-spans in which deterioration occurred in the three symptom dimensions - the manic dimension was excluded because of too small values. As Figures 5 and 6 show, only a small proportion of the patients experienced symptoms persisting over the entire follow-up period: 7% of the depressive symptoms, 6% of the negative and only 1% of the positive symptoms were of that type. The majority of exacerbations were of medium or short term, deterioration in the depressive core syndrome unfolding over 20.0 months (median 5 months), in the positive core syndrome over 6.3 months (median: 2) and in negative symptoms over 23.2 months (median: 5). This result, which reflects the high degree of interindividual variability, indicates that a clearly chronic course of any of the three syndromes, of the psychotic syndrome in particular, is extremely rare. The longest span of deterioration was shown by the depressive core syndrome, when the negative symptoms, not fully comparable, we-re left out of consideration.
These results strongly suggest that schizophrenia does not represent primarily a static encephalopathy46,47, but a dynamic process originating in a neurodevelopmental disorder. That process unfolds with bouts of increasing dysfunction followed by neuroplastic compensation permitting functional recovery. The process is characterised by asynchronous waves of exacerbation, triggered by intrinsic and extrinsic factors, and remission of its main symptom dimensions, a process we do not yet fully understand.
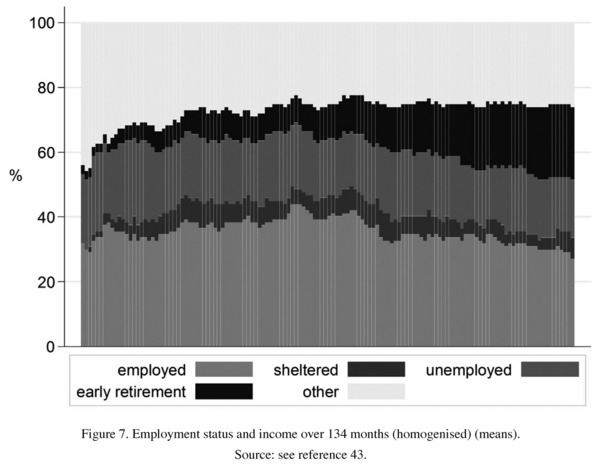
Social course was assessed on the basis of the proportion of patients in full-time employment, a well-comparable measure (Figure 7). At initial assessment the figure was about 30%, several years later it had increased to over 40% and at the final follow-up fallen back to 30%, compared with 70% for the healthy controls. On average, the disorder does not obviously lead to a defect state and dementia. However, social impairment is pronounced. The maximum of social decline or social stagnation occurs at the early stage of schizophrenia.
To conclude, the majority of the psychopathology currently diagnosed as schizophrenia involves both psychotic and depressive symptoms, with the latter being clearly more frequent. After illness onset different types of symptoms occur successively with depression leading the way in the early active stage characterised by an accumulation of symptoms and social consequences. The first episode is followed by asynchronous waves of exacerbation and intervals. The combination of positive, manic and negative symptoms and different proportions of concomitant depressive symptoms -the hierarchy of symptom frequencies remaining unchanged- makes up what is traditionally understood as schizophrenia. Since our current therapeutic instruments are not diagnosis-specific, i.e. not targeted at schizophrenia, but its syndromes, it should seriously be considered whether a specific therapy directed at current symptoms would not be more efficacious than the traditional diagnosis-based treatment. What we do already know is that focussing therapy and preventive measures exclusively on psychotic symptoms while neglecting the leading symptom dimension, depression, does not provide the best possible treatment. This regimen both ignores an increased risk for suicide and fails to relieve the distress caused by depression.
aBased on a paper presented at the International Symposium about Current Issues and Controversies "Beyond the Kraepelinean Nosology" held in Barcelona, April 3-4, 2008.
bDelusional perception; delusion of influence; experience of externally made actions, impulses and feelings; delusional mood.
References
1. Kraepelin E. Psychiatrie, 5th edn. Leipzig: Barth; 1896. [ Links ]
2. Kraepelin E. Die Erscheinungsformen des Irreseins. Zschr Ges Neurol Psychiat 1920; 62: 1-29. [ Links ]
3. Kendell RE, Brockington IF. The identification of disease entities and the relationship between schizophrenic and affective psychoses. Br J Psychiatry 1980; 137: 324-331. [ Links ]
4. Freyhan FA. Psychomotilität, extrapyramidale Syndrome und Wirkungsweisen neuroleptischer Therapien Nervenarzt 1957; 28: 504-509. [ Links ]
5. Owen MJ, Craddock N, O´Donovan MC. Schizophrenia: genes at last? Trends Genet 2005; 21: 518-525. [ Links ]
6. Owen MJ, Craddock N, Jablensky A. The genetic deconstruction of psychosis. Schizophr Bull 2007; 33: 905-911. [ Links ]
7. De Lisi LE, Faraone SV. When is a "positive" association truly a "positive" in psychiatric genetics? A commentary based on issues debated at the World Congress of Psychiatric Genetics, Boston, October 12-18, 2005. Am J Med Genet B Neuropsychiatr 2006; 141B: 319-322. [ Links ]
8. Li D, He L. Association study between the dystrobrevin binding protein I gene (DTNBP1) and schizophrenia: a meta-analysis. Schizophr Res 2007; 96: 112-118. [ Links ]
9. Li D, He L. G72/G30 genes and schizophrenia: a systematic meta-analysis of association studies. Genetics 2007; 175: 917-922. [ Links ]
10. Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C, et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry 2008; 165: 497-506. [ Links ]
11. Berrettini WH. Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry 2000; 48: 531-538. [ Links ]
12. Craddock N, O´Donovan MC, Owen MJ. Genetics of schizophrenia and bipolar disorder: dissecting psychosis. J Med Genet 2005; 42: 193-204. [ Links ]
13. Van den Bogaert A, Del-Favero J, van Broeckhoven C. Major affective disorders and schizophrenia: a common molecular signature? Hum Mutat 2006; 27: 833-853. [ Links ]
14. Hattori E, Liu C, Badner JA, Bonner TI, Christian SL, Maheshwari M, et al. Polymorphisms at the G72/G30 gene locus, on 13q33, are associated with bipolar disorder in two independent pedigree series. Am J Hum Genet 2003; 72: 1131-1140. [ Links ]
15. Detera-Wadleigh SD, McMahon FJ. G72/G30 in schizophrenia and bipolar disorder: review and meta-analysis. Biol Psychiatry 2006; 60: 106-114. [ Links ]
16. Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, et al. Genetic and physiological data implicatining the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 2002; 99: 13675-13680. [ Links ]
17. Williams NM, Green EK, Macgregor S, Dwyer S, Norton N, Williams H, et al. Variation at the DAOA/G30 locus influences susceptibility to major mood episodes but not psychosis in schizophrenia and bipolar disorder. Arch Gen Psychiatry 2006; 63: 366-373. [ Links ]
18. Hennah W, Thomson P, Peltonen L, Porteous D. Genes and schizophrenia: beyond schizophrenia: the role of DISC1 in major mental illness. Schizophr Bull 2006; 32: 409-416. [ Links ]
19. Craddock N, Owen MJ. Rethinking psychosis: the disadvantages of a dichotomous classification now outweigh the advantages. World Psychiatry 2007; 6: 20-27. [ Links ]
20. Kane JM, Lencz T. Cognitive deficits in schizophrenia: short-term and long-term. World Psychiatry 2008; 7: 29-30. [ Links ]
21. Van Os J, Janssen I, Hanssen M, Bak M, Myin-Germeys I, Marcelis M, et al. Cognitive epidemiology: psychological and social risk mechanisms for psychosis. In: Häfner H, editor. Risk and protective factors in schizophrenia. Darmstadt: Steinkopff; 2002; p. 207-228. [ Links ]
22. Van Os J, Krabbendam L, Myin-Germeys I, Delespaul P. The schizophrenia envirome. Curr Opin Psychiatry 2005; 18:141-145. [ Links ]
23. Selten JP, Cantor-Graae E, Kahn RS. Migration and schizophrenia. Curr Opin Psychiatry 2007; 20: 111-115. [ Links ]
24. W, Selten JP, Susser E, Laan W, Mackenbach JP, Hoek HW. Discrimination and the incidence of psychotic disorders among ethnic minorities in The Netherlands. Int J Epidemiol 2007; 36:761-768. [ Links ]
25. Ødegaard Ø. Emigration and insanity. A study of mental disease among the Norwegian-born population of Minnesota. Acta Psychiat (Kbh) 1932; Suppl 4. [ Links ]
26. Breier A, Schreiber JL, Dyer J, Pickar D. National Institute of Mental Health longitudinal study of chronic schizophrenia: prognosis and predictors of outcome. Arch Gen Psychiatry 1991; 48: 239-246. [ Links ]
27. Tsuang MT, Woolson RF, Fleming JA. Long-term outcome of major psychoses. I. Schizophrenia and affective disorders compared with psychiatrically symptom-free surgical conditions. Arch Gen Psychiatry 1979; 36: 1295-1301. [ Links ]
28. Marneros A, Deister A, Rohde A. Affektive, schizoaffektive und schizophrene Psychosen. Berlin: Heidelberg: Springer-Verlag; 1991. [ Links ]
29. Häfner H, Maurer K, Ruhrmann S, Bechdolf S, Klosterkötter J, Wagner M, et al. Early detection and secondary prevention of psychosis: facts and visions. Eur Arch Psychiatry Clin Neurosci 2004; 254: 117-128. [ Links ]
30. Häfner H, Maurer K, Löffler W, Riecher-Rössler A. The influence of age and sex on the onset and early course of schizophrenia. Br J Psychiatry 1993; 162: 80-86. [ Links ]
31. Häfner H, Nowotny B, Löffler W, an der Heiden W, Maurer K. When and how does schizophrenia lead to social deficits? Eur Arch Psychiatr Clin Neurosci 1995; 246: 17-28. [ Links ]
32. Wing JK, Cooper JE, Sartorius N. Measurement and classification of psychiatric symptoms: An instruction manual for the PSE and CATEGO Program. London: Cambridge University Press; 1974. [ Links ]
33. Andreasen NC. The scale for the assessment of negative symptoms (SANS). Iowa City: University of Iowa; 1983. [ Links ]
34. Biehl H, Maurer K, Jablensky A, Cooper JE, Tomov T. The WHO Psychological Impairments Rating Schedule (WHO/PIRS). I. Introducing a new instrument for rating observed behaviour and the rationale of the psychological impairment concept. Br J Psychiatry 1989; 155 (Suppl. 7): 68-70. [ Links ]
35. WHO - World Health Organization. Psychiatric Disability Assessment Schedule. Geneva: WHO; 1988. [ Links ]
36. Jung E, Krumm B, Biehl H, Maurer K, Bauer-Schubart C. Mannheimer Skala zur Einschätzung von sozialer Behinderung (DAS-M). Weinheim: Beltz; 1989. [ Links ]
37. Häfner H, Riecher-Rössler A, Hambrecht M, Maurer K, Meissner S, Schmidtke A, et al. IRAOS: an instrument for the assessment of onset and early course of schizophrenia. Schizophr Res 1992; 6: 209-223. [ Links ]
38. Häfner H, Löffler W, Maurer K, Riecher-RösslerA, Stein A. IRAOS. Interview für die retrospektive Erfassung des Erkrankungsbeginns und -verlaufs bei Schizophrenie und anderen Psychosen. Bern: Hans Huber Verlag; 1999. [ Links ]
39. Häfner H, Löffler W, Maurer K, Riecher-Rössler A, Stein A. IRAOS - Interview for the retrospective assessment of the onset and course of schizophrenia and other psychoses. Göttingen: Hogrefe & Huber; 2003. [ Links ]
40. An der Heiden W, Könnecke R, Maurer K, Ropeter D, Häfner H. Depression in the long-term course of schizophrenia. Eur Arch Psychiatry Clin Neurosci 2005; 255: 174-184. [ Links ]
41. Häfner H, Maurer K, Trendler G, an der Heiden W, Schmidt M, Könnecke R. Schizophrenia and depression: challenging the paradigm of two separate diseases - a controlled study of schizophrenia, depression and healthy controls. Schizophr Res 2005; 77: 11-24. [ Links ]
42. Häfner H, an der Heiden W, Maurer K. Evidence for separate diseases? Stages of one disease or different combinations of symptom dimensions? Eur Arch Psychiatry Clin Neurosci 2008; 258 (Suppl.2): 85-96. [ Links ]
43. Häfner H. Psychose, depression und manische Symptomatik - Leitsyndrome eigener Krankheiten oder Kontinuum? In: Möller H-J, Müller N, editors. Schizophrenie - Zukunftsperspektiven in Klinik und Forschung. Wien: Springer; 2010 (in print). [ Links ]
44. Häfner H, an der Heiden W, Behrens S, Gattaz WF, Hambrecht M, Löffler W, et al. Causes and consequences of the gender difference in age at onset of schizophrenia. Schizophr Bull 1998; 24: 99-113. [ Links ]
45. Häfner H, Maurer K, Löffler W, an der Heiden W, Munk-JArgensen P, Hambrecht M, et al. The ABC schizophrenia study: a preliminary overview of the results. Soc Psychiatry Psychiatr Epidemiol 1998; 33: 380-386. [ Links ]
46. Gold S, Arndt S, Nopoulos P, O´Leary DS, Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. Am J Psychiatry 1999; 156: 1342-1348. [ Links ]
47. Goldberg TE, David A, Gold JM. Neurocognitive deficits in schizophrenia. In: Hirsch SR, Weinberger D, editos. Schizophrenia, 2nd edition. Oxford: Blackwell Publishing; 2003; p. 168-184. [ Links ]
![]() Correspondence:
Correspondence:
Prof. Dr. Dr. h.c. mult. Heinz Häfner
Central Institute of Mental Health
J5 D-68159 Mannheim. Germany
Tel.: +49 621 1703 2951
Fax: +49 621 1703 2955
E-mail: heinz.haefner@zi-mannheim.de
Received: 6 July 2009
Accepted: 18 December 2009