Introduction
Methadone was synthesized in the 1940s. It is a pure synthetic opioid agonist with strong affinity for and activity at the µ-opioid receptors. It is marginally more potent than morphine and has a longer duration of action, but has a lower euphoric effect1. Thus, methadone is an alternative to morphine in that it has the same analgesic properties but has milder adverse effects2.
In 1964, it was first used in clinical rehabilitation programs for opiate addictions such as those associated with heroin. These programs were developed by a research team at the Rockefeller University of New York3 and are known as methadone maintenance programs (MMPs). They were developed after World War II to treat heroin addictions in response to the sharp increase in consumption and deaths related to this substance in the late 1950s and early 1960s in New York City4,5.
Methadone was the first opioid substitute employed in the United States, Australia, and Europe and continues to be the most widely used today. Its use has been clearly shown to reduce illicit heroin use, drug overdose deaths, frequency of injection, and HIV transmission6. It also improves the patients’ quality of life, including positive changes in health and in social and physical functioning7,8. It has been shown that oral methadone maintenance treatments are effective for individual patients, improve public health, and are cost-effective9,10.
In Spain, heroin use peaked in the 1980s11. The first Spanish regulations on the prescription and dispensing of methadone for the treatment of opiate dependence appeared in 1983. However, it was not until 1990 that the prescription criteria were standardised and methadone treatment became widespread. Since then, a series of laws on the regulation and implementation of MMPs have been passed12-16 and continue to be developed17.
In the CAM, the General Sub-Directorate for Addiction Health Care (SGAA) was incorporated into the General Directorate for the Coordination of Health Care by Decree 125/2017 of October 17 of the Governing Council, which establishes the organisational structure of the Madrid Health Service. The General Directorate assumed powers over “the planning and coordination of resources for treatment, rehabilitation, and reintegration available to the Public Administrations in the matter of drug dependence, as well as resources belonging to privately owned institutions that receive public funds within the Community for addiction health care tasks”.
The SGAA coordinates a network of centres and services in the CAM that provide health care to patients with drug use and dependence problems. They help around 22,000 people each year. Approximately 16% of these patients are prescribed methadone as an opiate substitute for the treatment of heroin-related addiction disorders.
As a result of the aforementioned organisational change, Resolution 189/201818 was implemented, which tasked a Hospital Pharmacy Service (HPS) with supplying methadone to the Centres for the Comprehensive Care of Drug Addiction Patients (CAIDs) of the Madrid Health Care Service SGAA. The aim of this Resolution was to centralize the acquisition, preparation, distribution, and dispensing of methadone by the HPS. This initiative represented a first step in changing the pharmacotherapeutic health care model for the treatment of the patients in the program. To date, between 3,000 and 5,000 patients are receiving methadone in the 27 CAIDs across the CAM.
In the first phase, methadone acquisition and production management had to follow the current distribution cycle, dosage, and personalized administration in each CAID.
In the second phase, treatments would be protocolised, standardised, and harmonised through the development of pharmacoclinical prescription and pharmaceutical care protocols. Health care staff and patients at the CAIDs would receive information and training strategies.
Given Resolution 189/201818 and the staged approach to the objectives, the HPS assumed its new responsibilities in March 2018. This was the first time that pharmacists specialised in Hospital Pharmacy had conducted this kind of work in the setting of addiction treatment in the Autonomous Community of Madrid. This approach provides a range of opportunities that will facilitate the utilization and optimization of existing resources as well as a change in the pharmacotherapeutic health care model by incorporating the competences, skills, and knowledge of specialist pharmacists in the healthcare process.
This article describes the process of the centralization of the acquisition, preparation, distribution, and dispensing of methadone in the HPS. This process will enable a change in the pharmacotherapeutic health care model for the treatment of MMP patients by incorporating specialist pharmacists into the health care process.
Methods
In the first phase of the project, the workflow followed prior to Resolution 189/201818 was analysed to acquire all the resources and materials needed to implement the Centralized Pharmacy Unit in the HPS.
A pharmacotechnical laboratory was set up in the facilities of the HPS and equipped with 500 mL, 1,000 mL, 2,000 mL, and 2,500 mL volumetric flasks, a precision scale, magnetic stirrers, magnets, 800 mL, 2,000 mL, and 3,000 mL beakers, glass rods, a desktop computer, and essential security measures, such as electronic access control and safe storage, given that the medication under consideration was a narcotic.
Subsequently, procedures for acquiring methadone were established to guarantee supply to the CAIDs. Methadone hydrochloride was acquired through the Spanish Drug Agency and methadone tablets were purchased from the supplying laboratory. Likewise, a new workflow, schedules, route, and security procedures were established to transport the drugs to the CAIDs. At the beginning of the program, methadone was purchased directly from the supplying laboratory. Subsequently, in compliance with the Public Administration Contracts Law, a standard procedure for the acquisition of methadone hydrochloride and methadone tablets was designed and contracted out through public tender.
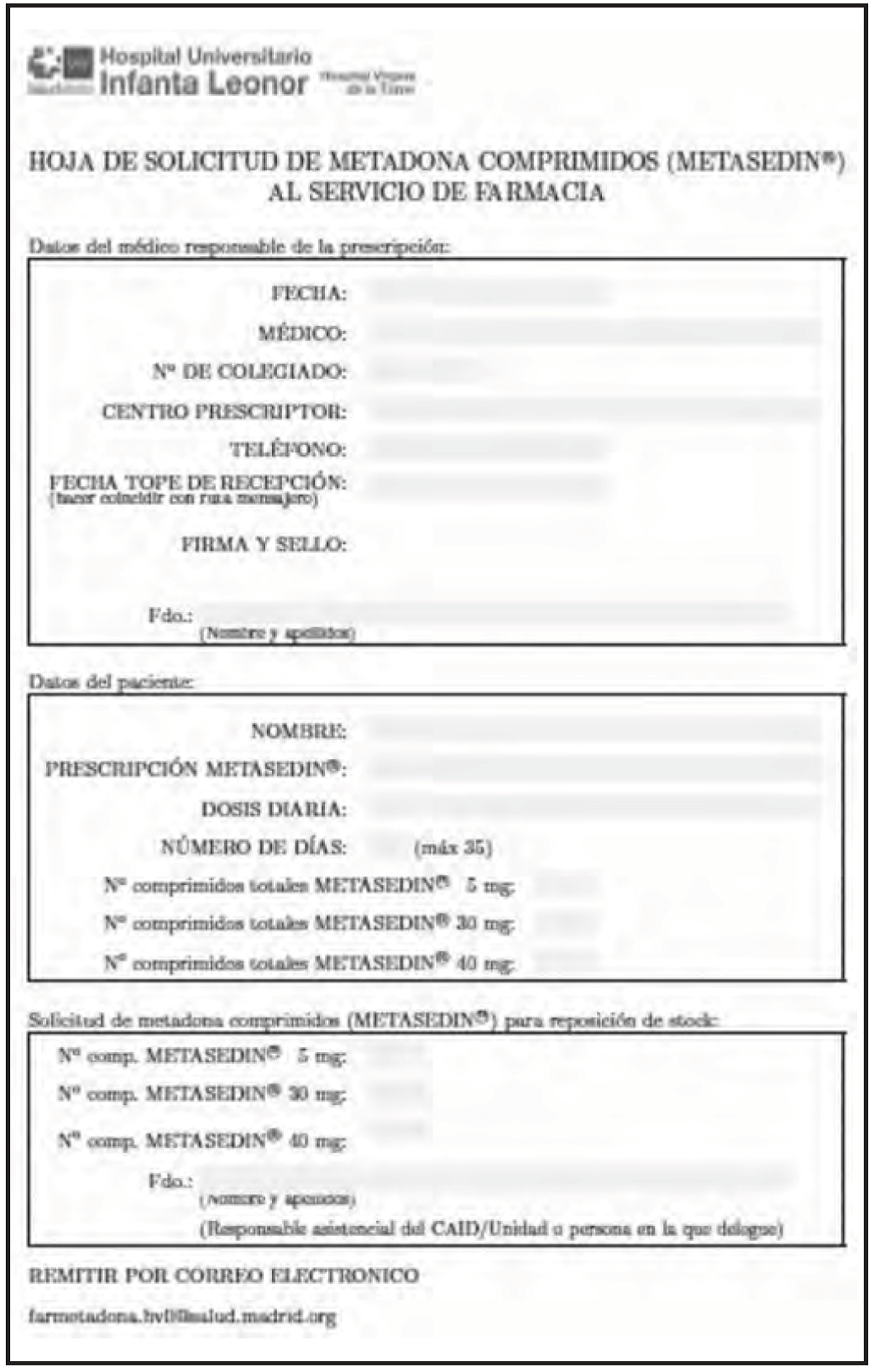
A communication system was set up between the HPS and the CAIDs and a new email account was created which was exclusive to this program. Two application forms were designed, one for oral methadone solution (Figure 1) and another for methadone tablets (Figure 2). The CAIDs use these forms to send their individualized requests to the HPS.
The HPS Quality Commission drafted and approved six standard operating procedures (SOPs) following the methodology implemented in the HPS in accordance with the ISO 9001:2015 Management System obtained in 2012.
The Pharmacy Service technical staff were trained in the theoretical and practical aspects of the program in order to incorporate them into their daily tasks.
Oral methadone solution
Methadone solution is prepared following the Good Practice Guideline for Drug Preparation in Hospital Pharmacy Services19 at a concentration of 10 mg/mL according to the National Formulary20. It is made in different volumes depending on the needs of the CAIDs.
Requests vary depending on the number of patients and population served by the CAID or the time of the year. These requests range from 5,000 mg to 200,000 mg of methadone hydrochloride (0.5-20 L). Thus, methadone solution is packaged in 0.5 L batches in order to be able to provide final packaging of 1 L or 2.5 L topaz glass bottles. During preparation, the batches are assigned according to the prescription book, and the corresponding preparation control sheet is completed. The batches dispensed to each CAID are recorded on the sheet, thus guaranteeing the traceability of the product. Once ready, the bottles are labelled, sealed with parafilm, and kept in a refrigerated room at 2 ºC to 8 ºC under security. The cold room is equipped with a sensor that monitors the temperature and its door remains locked until dispensing.
Methadone tablets
Methadone tablets are also dispensed to CAIDs according to individualized prescriptions made by the medical staff in each CAID. Each dose is packaged individually per patient and an information leaflet is included with each dose according to the pharmaceutical form of methadone.
Finally, a weekly work schedule was established in which four days were allotted for the preparation of methadone solution and one day for the preparation of individualized prescriptions for methadone tablets.
Transport and distribution
A standard procedure was designed for the transportation and distribution of methadone hydrochloride and methadone tablets. This procedure was contracted out through public tender in accordance with Public Administration Contracts Law.
Firstly, the transport route was created by assigning one day of the week to each CAID. Safety and security conditions were established. The containers are transported in flexible cooler bags with padded separators between the bottles to prevent impacts and breakage during transport. As a control and safety mechanism, a daily transport, delivery, and return control form was established between the HPS and the transport company workers. This form includes the number and volume of the methadone solution containers, total milligrams requested by each CAID, and whether or not individualized treatments with methadone tablets have been requested. The form also contains a final section in which the CAID stamps and confirms that the delivery has been completed correctly.
The HPS is also responsible for reviewing the accounts of each CAID through the daily dispensing records and accounting balance sheets, which are sent via e-mail, thus guaranteeing the correct use and control of the medication.
Daily workflow
Each CAID sends the request forms for methadone solution and methadone tablet prescriptions via email one week in advance. The data are anonymised by sending encrypted files with a separate key provided by the CAIDs. HPS administrative staff receive, record, and file requests. The pharmacist reviews and validates the requests and then organizes the weekly schedule. Methadone solution and individualized methadone tablet treatments are prepared according to the schedule. After the pharmaceutical validation of the compounding process, the route forms are filled in and two copies are made. Each day, the carrier collects the methadone according to the route sheet and weekly schedule. The carrier signs one of the copies, which is given to the administrative staff for its subsequent filing. The second copy is given to the carrier and the delivery cycle begins. Upon delivery, each CAID verifies that the methadone delivered corresponds to what has been requested. If everything is correct, the CAID stamps the delivery in the corresponding section of the form. This procedure is repeated at each CAID on the scheduled transport route, such that by the end of the day the form contains every CAID’s stamp. The carrier returns this form to the HPS and it is archived as proof that the methadone was correctly shipped and dispensed that day. In case of discrepancy, the CAID communicates with the HPS so that the issue can be resolved.
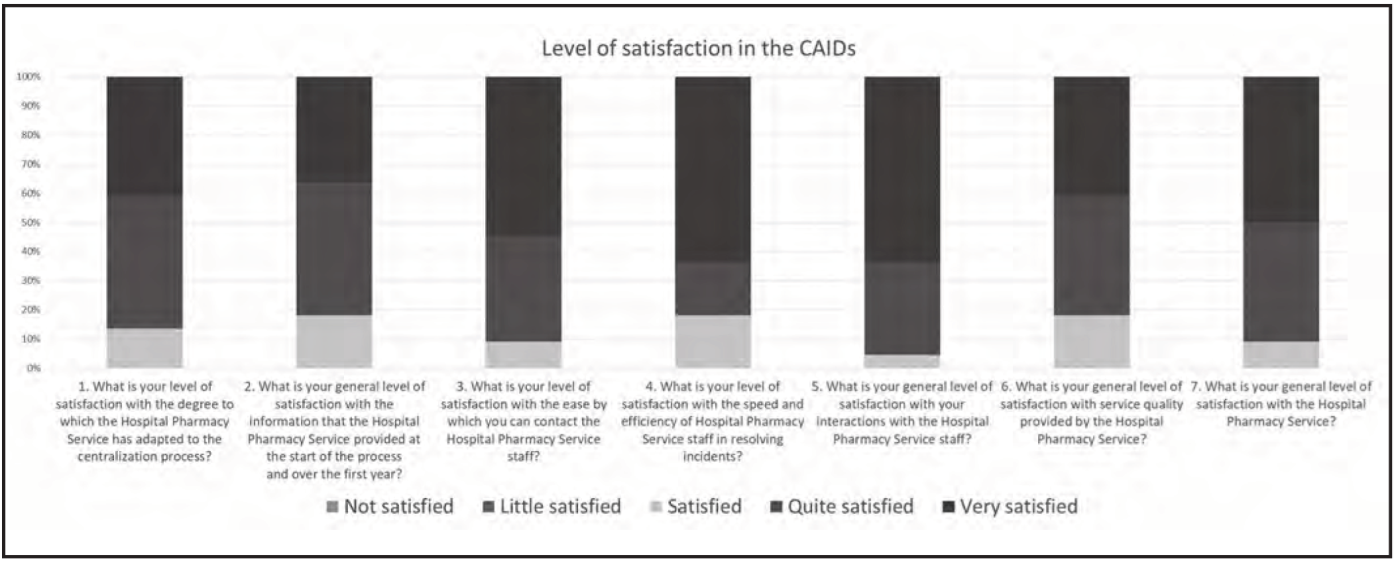
A questionnaire on perceived quality was designed to determine each CAID’s level of satisfaction with the centralization program. The questionnaire comprised a seven-item Likert-type scale (Table 1) with five possible replies: not satisfied, little satisfied, satisfied, quite satisfied, and very satisfied. It was sent to 24 CAIDs via email through the Google® Forms platform.
Results
Over the first year, standard procedures were designed for the acquisition of methadone, of containers for personalized dosing in the CAIDs, and for the transportation of methadone from the Hospital to the CAIDs. These procedures were contracted out through public tender in compliance with the Public Administration Contracts Law. The HPS pharmacist staff established all the technical specifications, needs, and assessment criteria.
The HPS serves a network of 27 CAIDs with between 3,000 and 5,000 patients in the MMP.
A total of 5,293.5 L of methadone solution has been produced since the beginning of the project between April 2018 and September 2019. Specifically, the HPS produced 76 1 L batches of methadone 10 mg/mL and 2,087 2.5 L batches of methadone 10 mg/mL (total, 5,217.5 L). Thus, approximately 53 kg (52,935 g) of methadone hydrochloride were consumed. Of the total produced, 5,242.75 L were dispensed.
A total of 2,844 individualized requests were dispensed comprising 151,579 methadone tablets in the form of 32,857 5 mg tablets, 62,164 30 mg tablets, and 56,558 40 mg tablets. Tablets were dispensed for the following reasons: the development of intolerance to methadone in solution, travelling for holidays or personal or work reasons, or showing good control and adherence.
There was a monthly average of 138.7 dispensed requests, although this value increased 3-fold in the summer months. This was because many patients changed from methadone solution to tablets to make travel easier or for holiday reasons.
The total purchasing cost was € 63,100.9 (methadone hydrochloride:
€ 43,993.8; methadone tablets: € 19,117.1).
In line with the legal requirements on the control of narcotic drugs (Royal Decree 1675/2012 of December 14), over the last 18 months 7,934 files containing the daily dispensing records and accounting balance sheets of all the CAIDs have been reviewed. This decree regulates official prescriptions and special requirements for the prescription and dispensing of narcotic drugs for human and veterinary use21.
In the second phase, a public tender was launched to acquire a software management system and automated methadone dispensing systems. It was awarded in March 2019 and is currently being implemented in the CAIDs. The pharmacotherapeutic protocol of the MMP of the CAM is being updated in collaboration with a multidisciplinary team made up of health care staff from the CAIDs, the HPS, the Subdirectorate of Pharmacy and medical products, and the Subdirectorate of Addictions.
In total, 22 CAIDs completed the questionnaire (response rate, 92%). Regarding the overall level of satisfaction, 50% were very satisfied, 41% were quite satisfied, and 9% were satisfied. The results are shown in Figure 3.
Discussion
In Spain, MMPs have been analysed in different healthcare settings, such as outpatient centres, drug addict care centres, and community pharmacies. Publications related to these MMPs vary by the care modality in each Autonomous Community. In general, they present the results from different perspectives (e.g. by analysing effectiveness, economic aspects, or perceived quality through patient satisfaction questionnaires8,11,17,22-32). However, these results are related to objectives that differ from those of this article in that they address MMPs. In contrast, we describe the centralization of acquisition, preparation, distribution, and dispensing of methadone to the CAIDs in the CAM and the role of the pharmacist in this process. Thus, the results of this article cannot be compared with previously published results, given the degree of implementation up to this point in time.
In the CAM, methadone can be dispensed from the CAIDs or from community pharmacies that are within the Madrid Pharmacy Offices MMP. This MMP was created in 1997 in collaboration by the Ministry of Health and Social Affairs (Anti-Drug Agency) and the Official College of Pharmacists of Madrid33.
However, the procedure for acquiring, preparing, distributing, and dispensing methadone to CAIDs had never been centralized in the CAM. In this Community, this pioneering experience has been conducted under the direction and responsibility of an HPS. This is the first time that specialist pharmacists have performed health care tasks in the setting of addictions. This has led to a number of benefits.
Firstly, the health resources of the Madrid Health Service have been optimized, due, on the one hand, to using the resources of the HPS with minimal adaptation, and on the other hand, to designing standard procedures for the acquisition of methadone, of containers for personalized dosing in the CAIDs, and for the transportation of methadone. These procedures were contracted out through public tender in compliance with the Public Administration Contracts Law. These initiatives have increased efficiency in the process, thus contributing to the sustainability of the health care system. Secondly, the reduction of inter-centre variability through the creation of standardized work procedures has facilitated the unification and homogenization of the processes.
The quality of the medicine has also been improved by centralizing the preparation of methadone solution in a single HPS. In addition to having better facilities, the HPS prepares master formulas following the Good Practice Guideline for Drug Preparation in Hospital Pharmacy Services19 and has a traceability system that covers the entire the manufacturing process. These actions have increased patient safety.
However, these processes must be planned in advance such that the HPS can guarantee supplies to all the CAIDs given the high number of patients included in the MMP and the large amount of methadone solution needed for preparation.
The results of the questionnaire on perceived quality show a high level of satisfaction in the CAIDs. It should be noted that no score less than satisfied has been recorded and the lowest response was “satisfied” and that the predominant response was “very satisfied”. Other outstanding results were the high level of satisfaction with the efficiency and speed of the resolution of incidents by the HPS staff (81.8% “very satisfied” or “quite satisfied”) and with the attention and treatment received from the HPS staff (95.4% “very satisfied” or “quite satisfied”).
The lack of a computerized system for prescribing, dispensing, and administration of methadone stands out as a limitation to the centralization of the MMP. Among other benefits, such a system would enable prescribing doctors, specialist pharmacists, and other staff in the CAID to completely trace prescriptions and provide them with an electronic medical record containing the clinical aspects and pharmacotherapeutic history of the patient. They would then have complete information on the effectiveness, safety, and efficacy of methadone treatment as well as adherence to treatment.
From this limitation arise our next challenges and improvements that we want to contribute, such as the implementation of a software that integrates all the processes related to the use of methadone. In addition, a process is already underway to further integrate specialist pharmacists in the multidisciplinary clinical teams caring for drug addiction patients, thus providing added value and improving health outcomes32. Specialist pharmacists and other Madrid Health Service staff are updating the pharmacotherapeutic protocol of the MMP of the CAM.
In addition, possible improvements to the formulation of methadone solution are currently being studied due its short period of stability20. One possibility is the addition of preservatives. The physicochemical and microbiological stability of the formula are also being studied in order to increase its “beyond use date”. These improvements will improve organizational aspects of the workflow and increase adaptability to the individual dispensing needs of patients.
In conclusion, we highlight that the entire first phase of the project has been consolidated, the initial objectives of Resolution 189/201818 have been fulfilled, and that this workflow can serve as a model for other autonomous communities. The centralization of the MMP in the HPS facilitates changes to the pharmacotherapeutic health care model by incorporating the competencies, skills, and knowledge of hospital pharmacists into the health care process of opiate-dependent patients. To the greatest extent possible, it also makes available the experience and resources needed to address this health care challenge, thus benefitting patients and other staff and contributing to the sustainability of the health care system.











 texto en
texto en