Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
International Microbiology
versión impresa ISSN 1139-6709
INT. MICROBIOL. vol.7 no.1 mar. 2004
| RESEARCH NOTE | |||
|
| |||
| Clostridium difficile pilot
Summary. Colonic infection with Clostridium difficile, leading to pseudomembranous colitis, is a common complication of antibiotic therapy, especially in elderly patients. It has been suggested that non-pathogenic probiotic bacteria might prevent the development and recurrence of C. difficile infection. This double-blind, placebo-controlled study examines the role of probiotic administration in the prevention of C. difficile-associated diarrhoea (CDAD) in elderly patients receiving antibiotic therapy. Consecutive patients (150) receiving antibiotic therapy were randomised to receive either a probiotic containing both Lactobacillus and Bifidobacterium or placebo for 20 days. Upon admission to hospital, bowel habit was recorded and a faecal sample taken. Trial probiotic or placebo was taken within 72 h of prescription of antibiotics, and a second stool sample was taken in the event of development of diarrhoea during hospitalisation or after discharge. Of the randomised patients, 138 completed the study, 69 with probiotics in conjunction with antibiotics and 69 with antibiotics alone. On the basis of development of diarrhoea, the incidence of samples positive for C. difficile-associated toxins was 2.9% in the probiotic group compared with 7.25% in the placebo-control group. When samples from all patients were tested (rather than just those developing diarrhoea) 46% of probiotic patients were toxin-positive compared with 78% of the placebo group. [Int Microbiol 2004; 7(1): 59- 62] Key words: Clostridium difficile · probiotic · antibiotic-therapy · diarrhoea | ||
| |||
| Estudio piloto de Clostridium difficile: efecto del aporte suplementario de probióticos sobre la incidencia de diarrea causada por C. difficile Resumen. La infección de colon por Clostridium difficile, que produce colitis pseudomembranosa, es una complicación frecuente en las terapias con antibióticos, especialmente en pacientes de la tercera edad. Se ha sugerido que las bacterias probióticas no patógenas podrían prevenir el desarrollo de la infección por C. difficile. Este estudio de doble ciego con control mediante placebos examina la influencia de la administración de probióticos en la prevención de diarrea asociada a C. difficile (CDAD) en pacientes de la tercera edad sometidos a terapia con antibióticos. Se escogieron al azar 150 pacientes consecutivos sometidos a terapia con antibióticos y se les administró aleatoriamente durante 20 días un probiótico que contenía Lactobacillus y Bifidobacterium o un placebo. Tras su ingreso hospitalario, se anotó su régimen intestinal y se tomó una muestra fecal. El probiótico o el placebo se administró durante las 72 h primeras del tratamiento con antibióticos, y se tomó una segunda muestra de heces en el caso de aparecer diarrea durante la hospitalización o tras el alta médica. De los pacientes escogidos, 138 completaron el estudio, 69 tratados con antibióticos y probióticos y 69 solamente con antibióticos. Entre los pacientes que tuvieron diarrea, se encontró un 2,9% de muestras positivas para la toxina asociada a C. difficile en el grupo tratado con probióticos, en comparación con el 7,25% detectado en el grupo control tratado con placebo. Cuando se analizaron muestras de todos los pacientes (no solamente los que tuvieron diarrea), un 46% de los pacientes tratados con probióticos dieron positivo para la toxina, en comparación con el 78% del grupo tratado con placebo. [Int Microbiol 2004; 7(1):59- 62] Palabras clave: Clostridium difficile · probiótico · terapia antibiótica · diarrea | Estudo piloto de Clostridium dificile: efeito da suplementação com probióticos sobre a incidência de diarréia causada por C. difficile Resumo. A infecção do cólon por Clostridium difficile, derivando em colite pseudomembranosa, é uma complicação comum nas terapias com antibióticos, especialmente em pacientes na terceira idade. Tem sido sugerido que as bactérias probióticas não patógenas poderiam ter um efeito preventivo sobre o desenvolvimento da infecção por C.difficile. Este estudo, duplamente cego, com controle mediante placebos, examina a influência da administração de probióticos sobre a prevenção de diarréia associada a C. difficile (CDAD) em pacientes da terceira idade submetidos à terapia com antibióticos. Foram escolhidos 150 pacientes submetidos à terapia com antibióticos e se administrou, aleatoriamente, durante 20 dias um probiótico que continha Lactobacillus e Bifidobacterium ou um placebo. Na admissão hospitalar foram anotados o regime intestinal do paciente e foram colhidas amostra fecal. Durante as primeiras 72 horas do tratamento com antibióticos foram administrados conjuntamente probiótico ou placebo e foi tomada uma segunda amostra de fezes, caso o paciente desenvolvesse diarréia durante ou após a alta médica. Dos pacientes escolhidos, 138 completaram o estudo, 69 tratados com antibióticos e probióticos e 69 somente com antibióticos. Dentre os pacientes que desenvolveram diarréia, foram encontradas 2,9% de amostras positivas para a toxina associada a C. difficile no grupo tratado com probióticos, em comparação com 7,25% detectado no grupo controle tratado com placebo. Quando foram analisadas as amostras de todos os pacientes (não somente os que desenvolveram diarréia), 46% dos pacientes tratados com probióticos apresentaram positividade para a toxina em comparação com 78% do grupo tratado com placebo. [Int Microbiol 2004; 7(1):59- 62] Palavras chave: Clostridium difficile · probiótico · terapia antibiótica · diarréia |
Introduction
Clostridium difficile is a gram-positive, anaerobic bacillus that colonises the human large intestine, and produces at least two exotoxins: toxin A, which is primarily an enterotoxin, and toxin B, a cytotoxin. Colonisation by this organism and subsequent infection occur in response to disruption of the stability of the indigenous microflora. The altered colonisation resistance frequently occurs following antibiotic therapy in hospitalised patients [12]. Finegold [4] claimed that all antimicrobial agents (with the exception of vancomycin and parenterally administered aminoglycosides) have been documented as pre-disposing patients to susceptibility to C. difficile infection. Responses to colonisation of the large intestine by C. difficile vary from asymptomatic, to mild diarrhoea, through to pseudomembranous colitis.
C. difficile is one of the most common causes of infectious diarrhoea in hospitals and nursing homes [10] and is the leading cause of nosocomially acquired intestinal infections in the USA [16]. Within hospitals, the extensive use of antibiotics, together with the inherent environmental contamination provides sources of cross-infection. Prevalence of C. difficile in the general environment is far lower than in health-care facilities. A single case of C. difficile-associated diarrhoea per 15,000 out-patients has been recorded, but up to 20% of in-patients may be colonised by C. difficile.
The financial burden associated with C. difficile infections is substantial for hospitals. Recurrence of symptoms following treatment of the infection is a particular problem with 5 to 66% of patients suffering from recurrences [16,18]. Jones and MacGowan [7] stated that, despite the issue of guidelines [DoH/PHLS, 1994 (Department of Health/Public Health Laboratory Service)] regarding the management and prevention of C. difficile infections, the problems continue and the authors suggested that the prophylactic use of biotherapies might be necessary to increase colonisation resistance.
Biotherapy (therapy involving probiotics) is emerging as a potential means of controlling C. difficile diarrhoeal recurrences, and promising results have been found when a stool sample was directly donated by colonoscopy [14] and when a non-pathogenic yeast (Saccharomyces boulardii) was used to treat C. difficile-infected rats and rabbits [2]. The role of the probiotic organisms is to restore the colonisation resistance of the normal flora, disrupted by the effects of antibiotic therapy, in order to prevent re-infection by C. difficile [9]. In this study, the emphasis is on the use of the probiotic product to prevent the initial infection and, thereby, minimise cross contamination and contain the spread of infection.
Materials and methods
Trial design. The trial was a double blind, placebo-controlled study in the departments of medicine and medicine for the elderly at Addenbrooke's Hospital, Cambridge. Patients with acute emergencies requiring treatment with antibiotics participated in the study. Ethical approval was obtained from the Cambridge Local Research Ethics Committee. Between March 1999 and July 2000, 150 patients were recruited and 138 patients fulfilled the inclusion criteria. For these patients, bowel habit on admission and prescribed medication were recorded.
Randomisation. Trial participants were randomised on arrival at hospital (probiotic group 69, placebo group 69) and each patient received one capsule/day for 20 days. The probiotic product (provided by Cultech, Swansea) comprised 2×1010 cfu Lactobacillus acidophilus and Bifidobacterium bifidum/capsule; the placebo comprised the inactive carrier. Trial treatments started within 36 h of antibiotic prescription (1.12 days for patients taking probiotics and 1.10 days for patients receiving placebo). Patients on a course of antibiotics lasting longer than 20 days were withdrawn from the trial, having had a final stool specimen collected.
Enumeration of Clostridium difficile. Faecal samples were enumerated following alcohol shock treatment. Faecal material was mixed with absolute alcohol (1:1, w/v), homogenised, and stored at 20ºC for 60 min. Dilution series set up anoxically in pre-reduced Maximum Recovery Diluent (MRD, Oxoid, Basingstoke, UK) and appropriate dilutions plated onto Clostridium difficile Agar (Oxoid) using a modified version (10×10 µl) of the Miles and Misra plate count method [11]. Growth was recorded after 48 and 72 h incubation at 37ºC. All presumptive C. difficile colonies were subcultured onto anoxic blood agar for Gram staining, and all obligate anaerobic gram-positive rods were tested to confirm that they were catalase-negative. Colonies were also tested using the Microscreen C. difficile Latex Slide Agglutination test (Microgen Bioproducts, Camberley, Surrey, UK) and/or API ID32A (Biomérieux, France). Samples positive for C. difficile were tested for the presence of C. difficile toxins A and B using an enzyme immunoassay kit (Ridascreen C. difficile toxin A/B, R-Biopharm, Darmstadt, Germany).
Statistical analysis. The trial was set up on the basis of a power calculation which estimated that, for an expected incidence rate of C. difficile infection of 10%, 400 patients would need to be recruited to show a 50% difference between the probiotic and the placebo in the prevention of C. difficile infection. The recruitment did not reach the required levels, which has made statistical analysis of the data limited. The data have been analysed using the methods of Newcombe [13] to determine confidence intervals for differences between proportions.
Results and Discussion
On arrival in hospital, patients were randomly allocated to receive probiotic or placebo in conjunction with their antibiotic therapy. Whilst on the ward, all episodes of diarrhoea were recorded, as is normal practice, and samples were sent to the hospital labs for typing. Unfortunately, it was not possible to achieve the required recruitment level during the course of this study so the final numbers were lower than had been indicated by the power calculation.
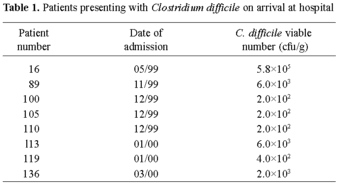
In addition to the standard hospital procedure of testing following the occurrence of diarrhoea, all participants in the trial provided faecal samples for testing at the start of the trial and following antibiotic therapy. On arrival at hospital, eight of the 138 participants (6%) were found to be carrying C. difficile asymptomatically (Table 1) with only one developing diarrhoea whilst in hospital (patient 16, who arrived with high numbers of C. difficile present). None of the patients tested positive for C. difficile toxin on arrival. This more detailed examination of the faecal samples from all patients (rather than exclusively for the diarrhoea patients) indicated that the numbers of patients carrying C. difficile was comparable in both groups, and such data (detailing presence of organisms) would not be detected using standard procedures in the hospital.
Finegold [4] suggested that up to 3% of healthy adults carry C. difficile, but many of the patients in this study may have received antibiotic therapy prior to hospitalisation or been hospitalised previously, which could have contributed to the elevated carrier status. Linevsky and Kelly [10] suggested that the asymptomatic carrier state may be due to toxin neutralisation rather than to the prevention of colonisation, as has been found in animals [8]. During this study, it appeared that there was an increase in C. difficile-associated problems following the admission of these asymptomatic carriers to hospital. In addition, the increase in the isolation rate of C. difficile from patients following antibiotic therapy clearly indicated the spread of this microorganism within the hospital environment.
Using the hospital-derived results to assess the 138 patients participating in the trial, 30 patients developed symptoms of diarrhoea (22% incidence rate), 15 patients in each treatment group. Analysis of the samples from these patients showed that five patients in the placebo group and two of the patients in the probiotic group tested positive for the presence of C. difficile toxin. Statistical analysis of these results indicated that the proportion developing diarrhoea positive for C. difficile-associated toxins was 4.35% lower in the probiotic group (95% CI of - 0.132 to 0.038).
There was a much greater proportion of patients positive for the toxins in the placebo group than in the probiotic group (which corresponds to the results obtained for diarrhoea patients from the hospital labs). In the placebo group, there appears to be a close relationship between the incidence of diarrhoea and the presence of toxin but this was less apparent among patients receiving probiotic. Thus, it would appear that many of the patients receiving the probiotic product were in the asymptomatic carrier state [10]. This may indicate the potential mode of action of the probiotic, i.e. by achieving some form of toxin neutralisation. Gorbach [6] found that administration of Lactobacillus GG resulted in an increase in the numbers of IgA- and other immunoglobulin-secreting cells in the intestinal mucosa, producing an enhanced immune response to the presence of C. difficile and/or its toxin. Such a response could account for the greater incidence of asymptomatic carriers observed among the probiotic group.
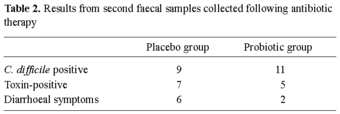
When the second faecal samples were analysed, C. difficile was detected in 20 of the 138 patients, four of whom had tested C. difficile-positive on arrival. Nine of the patients receiving placebo and 11 of the patients receiving probiotics were carrying this organism (Table 2). Toxin testing of the C. difficile-positive patients indicated that five of the 11 probiotic patients (46%) were toxin-positive with two of the five toxin-positive patients showing signs of diarrhoea. Seven of the nine placebo patients carrying C. difficile (78%) were toxin-positive, and six of these seven had diarrhoeal symptoms. Statistical analysis of the data obtained when all of the samples were analysed indicated a 32% difference between the detection of toxin among the C. difficile-positive placebo group patients and the probiotic group patients (95% CI of - 0.096 to 0.61). There appeared to be an increased incidence of C. difficile detection corresponding to the arrival of the asymptomatic carriers at the hospital.
The fact that, more C. difficile-positive patients were detected in the probiotic group than in the placebo group again may support toxin neutralisation rather than prevention of colonisation as the role of the probiotic organisms.
The participants in the trial were contacted following discharge, and 14 of the patients reported incidences of diarrhoea at home (9 placebo, 5 probiotic). Of these patients, however, only one had tested positive for the presence of C. difficile when the second faecal samples were analysed. In a trial with Lactobacillus GG, Pochapin et al. [15] found that, for a group of patients receiving either placebo or probiotic in conjunction with their antibiotic therapy, 30% (3/10) of the patients in the placebo group developed recurrent C. difficile-associated diarrhoea (CDAD) while none of the patients (0/6) in the probiotic group suffered recurrent CDAD. However, for patients who had previously suffered an episode of CDAD, the probiotic product did not appear to exert any beneficial effect.
When the medical and financial implications of C. difficile diarrhoea were considered, Eriksson and Aronsson [3] found that the median time for hospitalisation of the C. difficile patients was 50 days, compared with 14 days for uninfected controls. The mortality rates were 21% for the infected group and 7% for the control group (morbidity 14% and 4% respectively).
From the financial aspect, Spencer [17] suggested that the major cost implications associated with C. difficile infection/outbreaks related to increased hospital stay, antibiotic treatment, possible ward closure and loss of bed days together with infection control requirements. Wilcox et al. [18] estimated that the average additional length of stay in hospital was 21.3 days longer for C. difficile patients, corresponding to additional treatment costs per patient of £4,000. On the basis of the hospital-derived results in this study, the five placebo-group positive patients would have incurred an additional £20,000 expenditure whereas the probiotic group costs would have been £8,000. If it is assumed that administration of probiotic to all the patients in the trial incurred an additional cost of £2,000, the overall savings achieved from the probiotic supplementation could have been £10,000, a 50% reduction in costs.
In 1999, more than 15,000 cases of C. difficile were reported in the National Health Service (NHS), which would have cost more than £60 million to treat. If the findings of this pilot study can be confirmed by a more extensive study, treatment costs could be reduced by £30 million, and more than 300,000 hospital-bed days could be made available. If, with a larger study, the trends from this study are confirmed, the justification for the use of probiotic therapy for all patients receiving antibiotic therapy on admission to hospital would be clearly evident.
References
1. Biller JA, Katz AJ, Flores AF, Buie TM, Gorbach SL (1995) Treatment of recurrent Clostridium difficile colitis with Lactobacillus GG. J Pediatr Gastroenterol Nutr 21:224-226 [ Links ]
2. Castagliuolo I, Riegler MF, Valenick L, LaMont JT, Pothoulakis C (1999) Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect Immun 67:302-307 [ Links ]
3. Eriksson S, Aronsson B (1989) Medical implications of nosocomial infection with Clostridium difficile. Scand J Infect Dis 21:733-734 [ Links ]
4. Finegold SM (1986) Anaerobic infections and Clostridium difficile colitis emerging during antibiotic therapy. Scand J Infect Dis (Suppl) 49:160-164 [ Links ]
5. Gorbach SL, Chang T-W, Goldin B (1987) Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. Lancet 2:1519 [ Links ]
6. Gorbach SL (2000) Probiotics and gastrointestinal health. Am J Gastroenterol 95 (Suppl 1):2-4 [ Links ]
7. Jones EM, MacGowan AP (1998) Back to basics in management of Clostridium difficile infections. Lancet 352:505-506 [ Links ]
8. Kim PH, Iaconis JP, Rolfe RD (1987) Immunization of adult hamsters against Clostridium difficile associated ileocolitis and transfer of protection to infant hamsters. Infect Immun 55:2984-2992 [ Links ]
9. Kyne L, Kelly CP (2001) Recurrent Clostridium difficile diarrhoea. Gut 49:152-153 [ Links ]
10. Linevsky JK, Kelly CP (1997) Clostridium difficile colitis in gastrointestinal infections. In: LaMont, JT (ed) Gastrointestinal Infections: Diagnosis and Management. Marcel Dekker, New York, pp 293-325 [ Links ]
11. Miles AA, Misra SS (1938) The estimation of the bactericidal power of the blood. J Hygiene 38:732-749 [ Links ]
12. Naaber P, Mikelsaar RH, Salminen S, Mikelsaar M (1998) Bacterial translocation, intestinal microflora and morphological changes of intestinal mucosa in experimental models of Clostridium difficile infection. J Med Microbiol 47:591-598 [ Links ]
13. Newcombe RG (1998) Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med 17:873 - 890 (erratum in Stat Med 1999 May 30; 18:1293) [ Links ]
14. Persky SE, Brandt LJ (2000) Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol 95:3283-3285 [ Links ]
15. Pochapin M, Oltikar A, Pringle-Smith R, Schreiber C (1998) A prospective randomised placebo-controlled trial of Lactobacillus GG in combination with standard antibiotics for the treatment of Clostridium difficile infection. Am J Gastroenterol 93:1697 (Abstract) [ Links ]
16. Pochapin M (2000) The effect of probiotics on Clostridium difficile diarrhea. Am J Gastroenterol 95(Suppl 1):S11-S13 [ Links ]
17. Spencer RC (1998) Clinical impact and associated costs of Clostridium difficile-associated disease. J Antimicrob Chemother 41(Suppl C):5-12 [ Links ]
18. Wilcox MH, Cunniffe JG, Trundle C, Redpath C (1996) Financial burden of hospital-acquired Clostridium difficile infection. J Hosp Infect 34:23-30 [ Links ]