Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.5 sep. 2007
Dental treatment of patients with coagulation factor alterations: An update
Alba Jover Cerveró1, Rafael Poveda Roda2, José V. Bagán3, Yolanda Jiménez Soriano2
(1) DDS. Private practice. Valencia
(2) Physician and dentist. Staff physician of the Service of Stomatology, Valencia University General Hospital
(3) Physician and dentist. Head of the Service of Stomatology, Valencia University General Hospital
ABSTRACT
Hemostasia is a defense mechanism that protects vascular integrity, avoids blood loss, and maintains blood fluidity throughout the circulatory system. The biochemical processes leading to blood clot formation are complex, and alterations can appear at any point within the chain of events. While a range of alterations can affect the coagulation factors, some are more common than others in the general population, including congenital (hemophilia A and B, Von Willebrands disease) and acquired disorders (anticoagulant drugs). Such diseases require special consideration in the context of dental treatment, and therefore must be known to dental professionals. Interconsultation with the hematologist will provide orientation on the characteristics of the disease and on the best approach to treatment, including the need for replacement therapy, the application of local hemostatic measures, the modification of anticoagulant therapy, etc. In any case, the most important concern is the prevention of bleeding complications by compiling a detailed clinical history, with adequate planning of treatment, and taking special care to avoid soft tissue damage during the dental treatment of such patients. The dental surgeon must enhance awareness among patients and their relatives of the importance of correct oral hygiene, which will help avoid the need for invasive dental treatments and will reduce the number of visits to the dentist.
Key words: Hemostasia, coagulation factors, hemophilia, Von Willebrands disease, anticoagulant drugs, interconsultation, bleeding accidents, prevention.
RESUMEN
La hemostasia es un mecanismo de defensa cuya finalidad es conservar la integridad vascular y evitar la pérdida de sangre, a la vez que mantiene la fluidez de la sangre en todo el torrente circulatorio. Los procesos bioquímicos que conducen a la formación de coágulos son complejos y pueden producirse trastornos a cualquier nivel. Las alteraciones que afectan a los factores de la coagulación son múltiples, pero algunas de ellas se presentan con más frecuencia en la población: congénitas (hemofilias A y B, enfermedad de von Willebrand) y adquiridas (fármacos anticoagulantes). Estas patologías requieren consideraciones especiales en el tratamiento dental, por lo que el odontólogo debe conocerlas. La interconsulta con el hematólogo del paciente le informará sobre las características de la enfermedad y las pautas de tratamiento: necesidad de terapia sustitutiva, empleo de medidas hemostáticas locales, alteración de la pauta de tratamiento anticoagulante, etc. En cualquier caso, la medida más importante a tomar es la prevención de complicaciones hemorrágicas mediante la elaboración de una correcta y detallada historia clínica, la planificación adecuada de los tratamientos y prestando especial cuidado de no dañar los tejidos blandos orales durante la terapéutica dental. Es labor del odontólogo concienciar al paciente y a sus familiares de que una correcta higiene oral evitará la necesidad de tratamientos dentales invasivos y reducirá las visitas al odontólogo.
Palabras clave: Hemostasia, factores de coagulación, hemofilia, enfermedad de von Willebrand, fármacos anticoagulantes, interconsulta, accidentes hemorrágicos, prevención.
Introduction
Hemostasia is a defense mechanism that protects vascular integrity, avoids blood loss, and maintains blood fluidity throughout the circulatory system (1,2). When a blood vessel is damaged, rupture of the endothelial lining exposes the blood to proteins within the subendothelial tissue; this in turn triggers three different but overlapping mechanisms (3):
- Vasoconstriction (vascular phase). Immediately after damage to the vascular wall, reflex vasoconstriction occurs, reducing blood flow from the damaged vessel (1,4).
- Platelet plug formation (platelet phase). The blood platelets adhere to the exposed collagen fibers in the damaged vascular wall, and also to each other - thus forming a platelet plug (2).
- Fibrin production to stabilize and strengthen the platelet plug (plasmatic phase). Plasmatic coagulation involves the transformation of fibrinogen (soluble) into fibrin (insoluble) via the mediation of thrombin, a proteolytic enzyme generated as a result of the activation of prothrombin. The transformation of prothrombin into thrombin in turn takes place via two pathways, intrinsic and extrinsic - though in fact both pathways interact continuously. The intrinsic pathway is triggered by the activation of coagulation factor XII as a result of contact between the latter and the subendothelial tissues in the damaged zone. The extrinsic pathway in turn is triggered when the blood comes into contact with tissue thromboplastin released by the damaged tissues, resulting in the activation of coagulation factor VII. From this point onwards, a cascade of metabolic reactions occurs, involving the different coagulation factors - with the formation of thrombin as end result (Table 1).
The blood clot is finally dissolved during the fibrinolytic phase. When the damaged vessel wall is repaired, activated factor XII facilitates the conversion of an inactive molecule present in plasma into an active form called kallikrein. The latter in turn catalyzes the conversion of inactive plasminogen into the active molecule plasmin - an enzyme that digests fibrin to yield degradation products, and thus facilitates clot dissolution (3).
Laboratory tests
A number of tests are available for evaluating patients with coagulation disorders in order to diagnose the precise deficiency involved:
- Bleeding time: This test assesses the vascular and platelet phases of blood clotting. Although it is a functional test of limited sensitivity, it remains a good screening option (5).
- Activated partial thromboplastin time (aPTT): This test evaluates the intrinsic and common pathways of blood coagulation (2,5).
- Prothrombin time (PT): This parameter evaluates the extrinsic and common pathways (2,5), though it has been found to be inexact and variable, and the values obtained in different laboratories are not comparable due to the different thromboplastin sources used. Indeed, such differences have led to bleeding problems secondary to excessive anticoagulation based on erroneously low PT values (5). In order to standardize PT, the World Health Organization (WHO) in 1983 introduced the International Normalized Ratio (INR)(6), which is the ratio between the PT of the patient in seconds and a control PT standardized by means of the so-called International Sensitivity Index (ISI), which indicates the sensitivity of the thromboplastin used as reagent. In this context, human brain thromboplastin is designated the reference standard of 1.0. For a PT value within the normal range, INR = 1 (1,5-7).
- Specific factor tests.
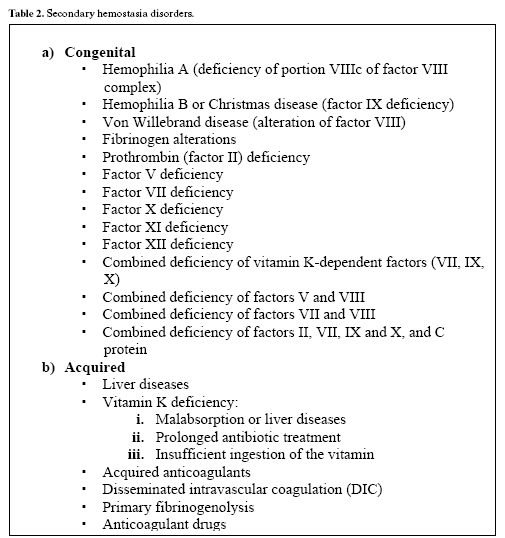
Table 2 reports the normal ranges for these parameters, together with the disorders in which the values can be altered.
Classification of coagulation factor disorders
The processes that lead to the formation of blood clots are complex, and alterations can take place at any level. The present review focuses on those alterations that affect the coagulation factors, and which can be classified as either congenital or acquired (Table 3). Due to the broad range of described alterations, we will limit in-depth examination to the most frequent disorders: hemophilia A and B and Von Willebrands disease among the congenital presentations, and anticoagulant drug administration in representation of the acquired disorders.
Congenital disorders
1.- HEMOPHILIA A
Definition and epidemiology. Hemophilia A is a hereditary bleeding disorder characterized by a deficiency in the activity of coagulation factor VIII (FVIII) in plasma, though with normal von Willebrand factor (FvW). The frequency in the general population is 1/5000 live born males (4,8,9).
Etiology and Pathogenesis. The disease shows X chromosome-linked hereditary transmission. As a result, women are able to carry the disease but do not suffer from it. The only exception is the rare case of a woman with a hemophiliac father and carrier mother (8,10).
Depending on the FVIII levels, three disease grades have been established: severe (< 1% of the normal levels), moderate (1-5% of the normal levels) and mild (5-50% of the normal levels)(4,5,8,11).
Clinical manifestations. The principal sign is bleeding; all other manifestations are a consequence of the latter (10).
Severe hemophilia is characterized by spontaneous bleeding of the joints and sometimes also of the soft tissues; any minor traumatism can lead to potentially life-threatening hemorrhage (5). The accumulation of bleeding episodes in the joints is associated with a high risk of developing joint disease. Bleeding within muscle tissue in turn can produce hematomas that cause pain, inflammation and dysfunction (8).
Diagnosis. The diagnosis is usually established from the clinical history. In this sense, hemophilia can be suspected from a family history of bleeding only in males, or from an exaggerated bleeding response to minor traumatisms and dental manipulations (1). The laboratory diagnosis is based on a very prolonged aPTT, while PT and bleeding time prove normal. The definitive diagnosis is established by quantitation of the procoagulant activity of FVIII, which is found to be reduced. A differential diagnosis must be established with Von Willebrands disease (10,11).
DNA analysis has made it possible to detect carriers and to establish a prenatal diagnosis in most cases (12). At present, preimplantation genetic diagnosis is possible, thus offering parents at risk of transmitting the disease the possibility of knowing whether the embryo is affected or not (13).
Treatment. The management options are practically limited to prophylaxis and the control of bleeding - where replacement therapy plays a preponderant role. Pharmacological antihemorrhagic treatment comprises antifibrinolytic agents and desmopressin (DDAVP), which are used when the bleeding manifestations are mild (10):
- Replacement therapy. Such treatment is based on the administration of antihemophilic factor concentrates. Human blood plasma products can be used to this effect, or alternatively plasma products obtained through recombinant technology (8,10). The half-life of FVIII is about 12 hours, which in turn determines the dosing interval (10).
The appearance of inhibitors that inactivate the function of the replaced factor is one of the most serious complications in the treatment of hemophilia. Thirty percent of all patients with severe hemophilia develop this problem (8).
- Desmopressin (DDAVP). This is a synthetic vasopressin analog that stimulates FVIII and FvW release from the endothelial cells and also increases platelet adhesion. Patients with detectable FVIII levels show a more predictable response to DDAVP, while those with undetectable levels of the factor fail to respond (14). Administration is usually via the intravenous route, though subcutaneous dosing or the inhalatory route (involving lesser response) also can be used. The administered dose is 0.3-0.4 μg/kg body weight as an intravenous infusion during 30 minutes, or as a subcutaneous injection. When the inhalatory route is chosen, the recommended dose is 300 μg in adults and 150 μg in children (14,15). The advantage is avoidance of the use of plasma concentrates (10,16).
- Antifibrinolytic agents. The two most widely used drugs are e-aminocaproic acid (EACA, Caproamin®) and tranexamic acid (AMCHA, Amchafibrin®). These drugs bind to the plasminogen binding site, resulting in the inhibition of fibrinolysis (14). The oral, intravenous or topical routes can be used, with the following doses: EACA 300 mg/kg/day in fractions every 4-6 hours; AMCHA 30 mg/kg/day in 2-3 daily doses (10,15).
- Gene therapy. The latest advances in gene therapy applied to hemophilia aim to correct the molecular defect in the mutant gene. At present, research is being done to add normal genes that encode for FVIII (or FIX in hemophilia B), based on recombinant technology (11,17).
Prognosis. With the currently available management options, the perspectives for patients with severe hemophilia have changed considerably. These individuals are now able to lead a normal life with few restrictions. On the other hand, gene therapy offers the possibility of true healing of the disease in animals - though application to humans is not yet possible (11).
Dental considerations. At oral level, the most frequent manifestations of hemophilia are prolonged, gingival bleeding episodes (either spontaneous or in response to trauma). Hemarthrosis of the temporomandibular joint is infrequent (1).
Dental management must center on prevention (measures of hygiene, fluor, sealing of cracks, dietary counseling and periodic controls) in order to reduce the need for dental treatment (18). However, if prevention is not possible and treatment is needed, the dental surgeon should contact the hematologist to know the specific characteristics of the disease in each individual patient, as well as the required factor replacement regimen based on the programmed dental treatment (1). In patients with mild to moderate hemophilia, noninvasive dental treatments can be carried out under antifibrinolytic coverage, while oral cleaning procedures and certain forms of minor surgery can be performed with DDAVP. In severe hemophilia, factor replacement is required (19), with the consideration of hospital admission. This decision is to be taken in coordination with the hematologist (5). The administration of local anesthetics is one of the main sources of concern, due to the risk of hematomas, airways obstruction and death. Anesthetic block or intramuscular injections are never carried out in the presence of FVIII levels of less than 50% the normal reference value, and in all cases they must be preceded by replacement therapy. Infiltrating pericemental and intrabony injections are to be preferred (5,12,18).
2.- HEMOPHILIA B
Definition and Epidemiology. Hemophilia B or Christmas disease is a congenital coagulation disorder caused by a quantitative or qualitative anomaly of coagulation factor IX (FIX)(1). The disease is five times less common than hemophilia A (8).
Etiology and pathogenesis. The gene encoding for FIX is located in chromosome X, i.e., the disease is sex-linked (1,12). In the same way as in hemophilia A, three grades of hemophilia B are observed: severe, moderate and mild (4,5,8,11).
Clinical manifestations. The disease is clinically indistinguishable from hemophilia A (8).
Diagnosis. The laboratory diagnosis is based on a very prolonged aPTT, while both PT and bleeding time are normal. The definitive diagnosis is established by quantitation of the procoagulant activity of FIX, which is found to be reduced (10,11).
Treatment. The principles of treatment are basically the same as in hemophilia A, and consist of FIX replacement therapy. The half-life of this factor is 24 hours, i.e., the dosing interval is longer than in hemophilia A, though the required dose is 25-50% greater than in the latter presentation of hemophilia (10). DDAVP is not useful in these patients (12). The generation of factor inhibitors is observed in less than 5% of cases (8).
Dental considerations. The guidelines for dental treatment are practically the same as in hemophilia A.
3.- VON WILLEBRANDS DISEASE
Definition and Epidemiology. Von Willebrands disease (vW disease) is considered to be the most common hereditary hemorrhagic disorder in humans (8,20), and is characterized by a prolonged bleeding time with low FVIII titers (4,12). It affects 1-2% of the general population (8,10,15). However, it is very likely that the application of stricter epidemiological criteria to differentiate between reductions in Von Willebrand factor (FvW) associated with mild or moderate bleeding risk and true Von Willebrands disease would reduce the prevalence (21).
Etiology and pathogenesis. Von Willebrands disease is characterized by FvW deficit or dysfunction. The main functions of this factor are the mediation of platelet adhesion and stabilization of FVIII in the bloodstream (4,10,15). As a result, FvW deficiency leads to a combined defect in platelet plug formation and fibrin formation (15).
Clinical manifestations. Mucosal membrane bleeding is characteristically observed (gingival hemorrhage, epistaxis, metrorrhagia), while hemarthrosis and musculoskeletal bleeding are only seen in the more severe forms of the disease (1). Women commonly present menorrhagia and, in the more severe presentations, postpartum bleeding (12).
Diagnosis. The usual coagulation tests are not sufficient for establishing the diagnosis of Von Willebrands disease. A number of specific tests therefore have been developed. In addition to bleeding time, other assays are performed: platelet agglutination induced by ristocetin, FvW titration, determination of FvW binding to collagen, determination of the antigenic activity of FvW, and evaluation of the multimeric structure of FvW (10).
Treatment. Management is based on DDAVP (desmopressin), which induces autologous secretion of FvW and FVIII from the endothelial cells, and plasma concentrates - which allogenically supplement the deficiency of these factors (15):
- DDAVP. Intravenous infusion is recommended for the treatment of acute bleeding, though the drug can also be administered via the subcutaneous or nasal route - which are preferred for prophylactic purposes (8,15). Dose testing is required to assess the response to treatment of each individual patient (10,15).
- Replacement therapy. This is indicated in patients that fail to respond to DDAVP or when the latter is contraindicated. Replacement therapy is also provided in cases of bleeding that prove life-threatening for the patient (8). 10-15% of all patients with severe forms of the disease develop autoantibodies against FvW antigen, thus creating complications for treatment (10,15).
On a complementary basis, use is also made of antifibrinolytic drugs (EACA and AMCHA) via the intravenous, oral or topical routes (8,10,15). In women, estrogens have been shown to be effective for the treatment of moderate menorrhagia (10).
Dental considerations. Prolonged bleeding is commonly seen after tooth extractions, as well as excessive bleeding associated with dental cleaning procedures (1).
Dental treatment must be individualized according to the severity of the condition, in coordination with the hematologist. In patients that respond to DDAVP, the latter is administered, while in refractory cases replacement therapy is used to reduce postoperative bleeding. The intranasal formulation is particularly useful for prevention in dental procedures, and has the advantage of allowing ambulatory administration. In addition to the above measures, antifibrinolytic agents can be administered (EACA, AMCHA), via the oral, intravenous or topical routes (1,8,9).
4.- OTHER COAGULATION FACTOR DEFICIENCIES
Congenital deficiencies of coagulation factors other than factors VIII (hemophilia A and vW disease) and IX (hemophilia B) are rare bleeding disorders (22). Their diagnosis and treatment pose important difficulties, due to the low frequency of these diseases in the general population (23).
Acquired disorders
1.- LIVER DISEASE. The liver synthesizes most of the coagulation factors, and is the source of the carboxylase needed for the g-carboxylation of the vitamin K-dependent factors. As a result, hepatocellular disease reduces the production of all the essential coagulation factors with the exception of FVIII and FvW, which are fundamentally produced by the endothelial cells (22).
2.- VITAMIN K DEFICIENCY. Prothrombin and factors VII, IX and X are produced in the liver cells, in processes requiring the presence of vitamin K (10). The latter intervenes in the g-carboxylation of these coagulation factors which in the absence of carboxylic acid addition are unable to bind to calcium, and are therefore functionally inert.
The most common causes of vitamin K deficiency (excluding the administration of anticoagulant drugs) are: malabsorption and liver diseases, prolonged antibiotic use (which eliminates the intestinal flora - a natural source of vitamin K2), and insufficient ingestion of the vitamin (12).
3.- ACQUIRED ANTICOAGULANTS. Acquired anticoagulants are type IgG autoantibodies targeted to one or more coagulation factors (10,12,24).
4.- DISSEMINATED INTRAVASCULAR COAGULATION (DIC). Disseminated intravascular coagulation is an acquired consumption coagulation disorder resulting from prolonged activation of the coagulation system. DIC is invariably the result of some underlying pathology, and must be evaluated in the context of the latter (12). Despite the disseminated production of fibrin, the main clinical problem of DIC is bleeding as a result of depletion (consumption) of the coagulation factors (10).
5.- PRIMARY FIBRINOGENOLYSIS. This disorder manifests when active plasmin is generated in the bloodstream while the coagulation cascade is inactive. Primary fibrinogenolysis can occur in patients with liver disease, lung cancer, prostate cancer and heat stroke. Severe bleeding occurs as a result of fibrinogen depletion (degraded by plasmin) and the formation of fibrin degradation products from fibrinogen, which have anticoagulant properties (5).
6.- ANTICOAGULANT DRUGS. Many bleeding tendencies are due to the use of anticoagulants, which are commonly prescribed for underlying cardiological diseases such as ischemic heart disease, heart valve problems and the implantation of valve prostheses, deep venous thrombosis, pulmonary embolism, and cerebrovascular accidents such as stroke (1,9,25).
Anticoagulation is provided with unfractionated or standard heparin, low molecular weight heparin (LMWH) and oral anticoagulants (coumarins)(2,25).
Standard heparin (SH). Standard heparin fundamentally acts as a catalyzer of plasmatic antithrombin III (ATIII)(1,26,27). The latter regulates coagulation, inhibiting certain factors of the coagulation cascade, and heparin binds to it to reinforce such inactivation (1,26).
SH is used at high doses for the treatment of thromboembolism, and at low doses to prevent the latter. It is generally used as an intravenous infusion in hospitalized patients, and requires aPTT monitorization. The only patients subjected to high dose SH treatment on an ambulatory basis are those on hemodialysis, though the effects last only a few hours after dialysis, since the half-life of SH is 1-2 hours (Figure 1).
Low molecular weight heparin (LMWH). This form of heparin exerts the same action as SH, and is used in place of the latter in patients programmed for major surgery, for the prevention of deep venous thrombosis - administration being via the abdominal subcutaneous route. LMWH is also used in the treatment of outpatients (25,26). It offers a number of advantages over SH. In effect, its longer half-life of 2-4 hours (26) allows for more predictable dosing, with a lesser need for monitorization, and a reduction in the incidence of heparin-induced thrombocytopenia (9). Moreover, the risk of postoperative bleeding is lower, since LMWH exerts a lesser effect upon the platelets (25), and its efficacy in preventing thromboembolism is greater (26).
Coumarin drugs. These substances comprise warfarin (Aldocumar®) and acenocoumarol (Dicumarol®, Sintrom®), which exert an anticoagulative effect as a result of vitamin K antagonism and the inhibition of vitamin K-dependent coagulation factor synthesis (factors VII, IX, X and prothrombin)(1). They are used at low doses for the treatment or prevention of venous thrombosis, and at higher doses in patients with heart valve prostheses, or as prevention against recurrent myocardial infarction.
Patients treated with oral anticoagulants require periodic controls (2). Monitorization is based on PT, since the latter is sensitive for three of the vitamin K-dependent coagulation factors: VII, X and prothrombin. As has been mentioned above, PT is imprecise, and use of the INR is presently recommended (26). The recommended anticoagulation levels are equivalent to an INR value of between 2 and 3 for all indications, with the exception of cardiac valve prostheses - where the INR should be kept between 2.5 and 3.5 (1,2,28)(Figures 2a and 2b).
Dental considerations. Most patients treated with SH are hospitalized, and once discharged, treatment is continued with coumarins. If a dental emergency arises during hospital admission, and conservative management is not possible, the supervising physician should be consulted (26), to decide whether SH can be suspended in order to carry out the dental procedure once its effect has disappeared. If emergency intervention proves necessary, the effect of SH can be countered by administering its antagonist, protamine sulfate, at an intravenous dose of 1 mg/100 IU of heparin (25-27). In dialyzed patients, and since SH has a half-life of 1-2 hours, it suffices to carry out any necessary invasive dental treatment on the day after dialysis (26). Outpatients administered LMWH can undergo invasive dental care without changes in medication, and postoperative bleeding can be controlled by the adoption of local measures. If heavier bleeding is anticipated, the anticoagulant treatment can be suspended for a day, with dental treatment taking place the day after (26).
There is controversy in the literature regarding the management of patients treated with coumarins (9). The great majority of authors consider that there is no need to modify anticoagulant therapy provided the INR is 4 or lower, since possible bleeding can be controlled with local measures, while the interruption of such medication does not necessarily reduce bleeding and moreover entails the risk of serious thromboembolic complications (7,25,26,29). In the case of important bleeding, vitamin K can be administered to revert the effect of medication, though prior consultation with the supervising physician is required in all cases (7). Lastly, consideration is required of the possible interactions of these drugs with other medications which the patient may be using, since the anticoagulant effect could be increased or reduced as a result (25,26).
Considerations for dental treatment
The most important objective is the prevention of complications. The following principles apply in this sense:
1. Identification of the patient based on a thorough clinical history: disease antecedents, exploration and laboratory testing for selective identification (1).
2. Counseling of patients and their relatives to increase awareness of the importance of good oral hygiene in order to avoid the need for invasive dental care and reduce the number of visits to the dentist (1,19).
3. Consultation with the specialist to determine the type of congenital disorder involved, the need or not for replacement therapy and the possible existence of inhibitors, or to obtain information on the degree of anticoagulation of patients subjected to anticoagulation therapy, and the need or not for dose reduction to ensure sufficient hemostasia (5,25).
4. Replacement therapy in necessary cases. Replacement may comprise coagulation factors (hemophilia A and B, and vW disease) or vitamin K (lack of ingestion or poor absorption and liver disease)(5,12).
5. Avoidance of brusque maneuvers during dental treatment, in order to prevent oral mucosal damage that may give rise to postoperative bleeding problems (1,12,19).
6. Evaluation of the advisability of hospital admission when complex surgery is required. In the case of hemophilia, the ideal approach would be to provide dental care in the specialized hemophilia center by dental surgeons integrated within the team supervising the patient (9).
7. Aspirin and its derivatives are to be avoided as pain treatment. In this sense, paracetamol is a safe alternative (1,18). In the case of the coumarins, the possibility of multiple interactions with other drugs must be taken into account - resulting in either enhancement of the anticoagulant effect (with the resulting risk of excessive bleeding), or a reduction of coumarin effects (with the risk of thromboembolic events). Some of these drugs are often prescribed in dental practice, including antibiotics (amoxicillin and amoxicillin plus clavulanate, ampicillin, azithromycin, erythromycin, rifampicin, penicillin G, cephalosporins, sulfonamides, metronidazole, chloramphenicol), antifungals (azoles and griseofulvin), analgesics (aspirin and other nonsteroidal antiinflammatory drugs; paracetamol in excess can enhance warfarin action), and psychoactive drugs (some antihistamines, diazepam)(25,26).
8. Reabsorbable sutures are recommended, in order to avoid the risk of bleeding associated with suture removal (1,18).
9. Hemophiliacs treated with human plasma derivatives may be carriers of hepatitis B or C viruses, HIV, parvovirus or transmissible spongiform encephalopathy (1,8,11).
10. Local hemostatic measures are recommendable: mechanical (sutures, compression, splints for protecting the clot), chemical agents (thrombin) or reabsorbable hemostatic products (oxidized-regenerated cellulose, microfibrilar collagen, fibrin sponges or gelatin plugs) (7).
References
1. Quintero-Parada E, Sabater-Recolons MM, Chimenos-Küstner E, López-López J. Hemostasia y tratamiento odontológico. Av Odontoestomatol 2004;20:247-61. [ Links ]
2. Poveda-Roda R. Enfermedades sistémicas y odontología. En: Bagán JV, Scully C, eds. Medicina y Patología Oral. Valencia: Medicina Oral SL; 2006. p. 285-302. [ Links ]
3. Fox SI. Fisiología Humana. Madrid: McGraw- Hill/Interamericana de España S.A.U.; 2002. p. 385-8. [ Links ]
4. Gómez-Moreno G, Cutando-Soriano A, Arana C, Scully C. Hereditary blood coagulation disorders: management and dental treatment. J Dent Res 2005;84:978-85. [ Links ]
5. Little JW, Falace DA, Miller C, Rhodus NL, eds. Tratamiento odontológico del paciente bajo tratamiento médico. Madrid: Harcourt SL; 2001. p. 466-92. [ Links ]
6. Stern R, Karlis V, Kinney L, Glickman R. Using the international normalized ratio to standarize prothrombine time. JADA 1997;128:1121-2. [ Links ]
7. Herman WW, Konzelman JL, Sutley SH. Current perspectives on dental patients receiving coumarin anticoagulant therapy. JADA 1997;128:327-35. [ Links ]
8. Brown DL. Congenital Bleeding disorders. Curr Probl Pediatr Adolesc Health Care 2005;35:38-62. [ Links ]
9. Schardt-Sacco D. Update on coagulopathies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:559-63. [ Links ]
10. Sans-Sabrafen J, Besses C, Vivens JL, eds. Hematología clínica. Madrid: Ediciones Harcourt SL; 2001. p.640-74. [ Links ]
11. Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. The Lancet 2003;361:1801-9. [ Links ]
12. Rose LF, Kaye D, eds. Medicina interna en odontología. Barcelona: Salvat editores SA; 1992. p.439-64. [ Links ]
13. Lavery S. Preimplatation genetic diagnosis: new reproductive options for carriers of haemophilia. Haemophilia 2004;10(Suppl 4):126-32. [ Links ]
14. Villar A, Jiménez-Yuste V, Quintana M, Hernández-Navarro F. The use of haemostatic drugs in haemophilia: desmopressin and antifibrinolitic agents. Haemophilia 2002;8:189-93. [ Links ]
15. Mannucci PM. Treatment of von Willebrands disease. N Engl J Med 2004;351:683-94. [ Links ]
16. Hay CR, Brown S, Collins PW, Keeling DM, Liesner R. The diagnosis and management of factor VIII and IX inhibitors: a guideline from de United Kingdom Haemophilia Centre Doctors Organisation. Br J Hematol 2006;133:591-605. [ Links ]
17. Pipe SW, Saint-Remy JM, Walsh CE. New high-technology products for the treatment of haemophilia. Haemophilia 2004;10:56-63. [ Links ]
18. Brewer A, Corre ME. Directrices para el tratamiento odontológico de pacientes con trastornos de la coagulación hereditarios. Serie monográfica. El tratamiento de la hemofilia. Montreal, Canadá: Federación Mundial de Hemofilia; 2006. [ Links ]
19. Scully C, Diz-Dios P, Giangrande P, Lee C. Cuidados orales para personas con hemofilia u otras alteraciones hereditarias de la coagulación. Serie monográfica. El tratamiento de la hemofilia. Montreal, Canadá: Federación Mundial de Hemofilia; 2002. [ Links ]
20. Federeci AB. Clinical Diagnosis of von Willebrand disease. Haemophilia 2004;10:169-76. [ Links ]
21. Batlle-Fonrodona J, Noya-Pereira MS, Pérez-Rodríguez A, Lourés-Fraga E, López-Fernández MF. Tratamiento de la enfermedad de von Willebrand y su monitorización. Hematologica 2004;89:10-9. [ Links ]
22. Litchtman MA, Beutler E, Kipps TJ, Williams WJ, eds. Manual Williams de Hematología. Madrid: Marban libros SL; 2005. p. 461-83. [ Links ]
23. Bolton-Maggs, PH Perry DJ, Chalmers EA, Parapia LA, Wilde JT, Williams MD et al. The rare coagulation disorders – review with guidelines for managements from the United Kingdom Haemophilia Centre Doctors Organisation. Haemophilia 2004;10:593-628. [ Links ]
24. Collins P. Acquired hemophilia. Semin Hematol 2006; 43:90. [ Links ]
25. Scully C, Wolff A. Oral surgery in patients on anticoagulant therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:57-64. [ Links ]
26. Little JW, Miller CS, Henry RG, McInstosh BA. Antitrhombotic agents: implications in dentistry. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;93:544-51. [ Links ]
27. Mehra P, Cottrell D, Bestgen SC, Booth DF. Management of heparin therapy in the high-risk, chronically anticoagulated, oral surgery patients: a review and a proposed nomogram. J Oral Maxillofac Surg 2000;58:198-202. [ Links ]
28. Wahl MJ, Howell J. Altering anticoagulation therapy: a survey of physicians. JADA 1996;127:625- 38. [ Links ]
29. Campbell JH, Alvarado F, Murray RA. Anticoagulation and minor oral surgery: should the anticoagulation regime be altered?. J Oral Maxilofac Surg 2000;58:131-5. [ Links ]
30. Vicente-Barrero M, Knezevic M, Tapia-Martin M, Viejo-Llorente A, Orengo-Valverde JC, Garcia-Jimenez F et al. Oral surgery in patients undergoing oral anticoagulant therapy. Med Oral 2002 ;7:63-70. [ Links ]
![]() Correspondence:
Correspondence:
Dra. Alba Jover Cerveró
C/ Ribera Alta nº 6
46192 Montserrat (Valencia)
E-mail: albajover@hotmail.com
Received: 5-11-2006
Accepted: 25-03-2007