Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.7 nov. 2007
Oral non-squamous malignant tumors; diagnosis and treatment
Rutger van der Waal1, Isaäc van der Waal2
(1) MD, PhD
(2) DDS, PhD. I. Professor of Oral Pathology. Head of the Department of Oral and Maxillofacial Surgery/Oral Pathology. Vrije Universiteit University Medical Center/Academic Center for Dentistry Amsterdam. Amsterdam, the Netherlands
ABSTRACT
Some 90% of oral cancers consist of squamous cell carcinomas that arise from the oral mucosa. The remaining 10% of malignancies consist of malignant melanomas, carcinomas of the intraoral salivary glands, sarcomas of the soft tissues and the bones, malignant odontogenic tumors, non-Hodgkins lymphomas and metastases from primary tumors located elsewhere in the body. These malignancies will be briefly reviewed and discussed. The emphasis is on diagnosis and management.
Key words: Oral cancer; oral sarcoma, oral malignant melanoma, oral metastases.
Introduction
Malignancies of the oral cavity largely (90%) consist of primary squamous cell carcinoma arising from the mucosal lining. Tobacco and excessive use of alcohol play an important role in the aetiology of these cancers. The remaining 10% of oral malignancies consist of a heterogeneous group of tumors of unknown aetiology, and comprise malignant melanoma, malignant intraoral salivary gland tumors, sarcomas of the soft tissues and the jaw bones, non-Hodgkins lymphomas, and the rare oral metastases of primary tumors located elsewhere in the body. These oral non-squamous malignant tumors will be briefly discussed, the emphasis being on diagnosis and management.
Oral malignant melanoma
Approximately 1% of all malignant melanomas arise in the oral mucosa. There are no known aetiologic factors. Oral pigmented nevi do not seem to be risk markers of future development of an oral malignant melanoma.The patients are usually above the age of 40 years. There is no distinct gender preference. The palate and the upper and lower gingival are the sites of preference (1).
The clinical presentation of a malignant melanoma is usually a brown-black coloured swelling with or without ulceration (Fig. 1). Occasionally, there is no distinct melanin pigmentation, being referred to as an amelanotic melanoma. The diagnosis requires a biopsy. In case of suspicion of a cutaneous melanoma usually an excisional biopsy is recommended, since it has been suggested that an incisional biopsy may enhance the risk of metastatic spread (2). In the oral cavity an excisional biopsy is often not feasible because of the presence of teeth or bones.
The histopathologic features can be misleading, particularly if melanin pigment is not abundant. In such cases tumor markers such as HMB45 may be helpful to identity the nature of the tumor.
There is a clinical staging system for head and neck malignant melanomas, including oral malignant melanomas, that recognizes three stages; furthermore, stage I can be subclassified in three levels (Table 1) (3).
Treatment usually consists of wide surgical excision. The role of postoperative radiotherapy is questionable. Adjuvant chemotherapy does not seem to influence survival (4). Systemic immunotherapy with IL-2 and other cytokines is not associated with an improved survival rate either (4). The detection of the so-called cancer testis antigens (CTAs) expression profile in oral malignant melanoma could lead to the development of a new vaccine-based therapy (5). Prognosis is usually poor due to local recurrences and metastatic spread, either through the lymph vessels or the blood stream. Clinical stage at presentation is probably the most important factor in determining outcome (6). In a study on 230 patients surgically treated for roal malignant melanoma, thickness of the tumor, cervical lymph node metastasis, presence or absence of ulceration and the anatomic sites were all independent risk factors (7).
Malignant melanoma is probably often preceded by diffuse areas of seemingly benign melanotic pigmentation, also referred to as melanosis. It is questionable whether treatment of melanosis is effective in preventing the development of a malignant melanoma.
Tumors of the intraoral salivary glands
Tumors of the intraoral salivary glands are relatively rare. The aetiology is unknown. The estimated incidence of benign and malignant intraoral salivary gland tumors together is less than 1 per 100.000 population per year. The patients are usually above the age of 20 years. There is no distinct gender preference.
The posterior part of the palate is the site of predilection, followed by the upper lip. Occurrence of a salivary gland tumor in the floor of the mouth is extremely rare. Interestingly, such salivary gland tumors in this location are nearly almost of a malignant type. Intraoral salivary gland tumors rarely occur in the jaws. If they do, it is usually the mandible.
The clinical presentation of both a benign and a malignant intraoral salivary gland tumors is often a slow growing, otherwise asymptomatic swelling with no characteristic features (Fig. 2). In other words, the nature of a salivary gland tumor, being benign or malignant, can not reliably be predicted on the clinical presentation alone. Usually preference is given to an incisional biopsy above fine needle aspiration cytology to arrive at a proper preoperative diagnosis. Only in well-circumscribed nodules in the lips or the cheek mucosa an excisional biopsy may be considered. Approximately 50% of all intraoral salivary gland tumors are malignant. One of the common malignant intraoral salivary gland tumors is the adenoid cystic carcinoma.
In 2005 the World Health Organisation has issued a revised histopathological classification (8). Histopathologic typing can at times be difficult, even more so in tumors of the minor salivary glands than in tumors of the major glands. There are several histopathological pitfalls, such as necrotising sialometaplasia and metastatic tumors that may mimic salivary gland tumors. Tumor markers are in general of limited help in the histopathologic classification of salivary gland tumors. Grading of such tumors is of limited value with the possible exception of mucoepidermoid carcinomas.
Treatment consists of surgical removal and, on indication, of postoperative radiotherapy. Prognosis mainly depends on stage and histological type.
Sarcomas of the oral soft tissues (excluding Kaposis sarcoma)
The estimated annual incidence of sarcomas of the oral soft tissue is approximately one per million population. They constitute less than one per cent of all oral malignancies. There are no known aetiologic factors. Soft tissue sarcomas may occur at all ages. There is no gender preference.
The clinical presentation is not characteristic and usually consists of a firm elastic swelling (Fig. 3). There is a wide variety of histological types, such as fibrosarcoma, liposarcoma, rhabdomyosarcoma. In 2002 a revised classification of soft tissue tumors has been published by the WHO (9). Numerous types and subtypes have been identified in that classification. Many of these are difficult to classify histopathologically with certainty in spite of the use of tumor markers. Furthermore, some of these tumors are classified as intermediate tumors, between the category of benign and malignant ones, since metastases may occasionally occur in such tumors, e.g. in solitary fibrous tumor and hemangiopericytoma. Histopathological grading, taking into account tumor differentiation, mitotic counts and tumor necrosis, evaluates the degree of malignancy and the probability of distant metastases (9). In general, three groups are recognised, high, intermediate, and low grade malignancy. Staging, based on both clinical and histopathological parameters, provides information on the extent of tumor.
Treatment usually consists of wide surgical excision with or without (neo)adjuvant chemotherapy. In deeply located soft tissue sarcomas or in case of irradical removal of a soft tissue sarcoma, postoperative radiotherapy is often used, although no evidence-based data about the efficacy of such treatment is available. Prognosis mainly depends on stage and histological type (10, 11).
Osteosarcomas of the jaws
Approximately 5% of all osteosarcomas that may occur in the skeleton arise in the jaws. Chondrosarcomas rarely affect the jaw bones. Osteosarcomas may affect children and young adults (12). There is no distinct gender preference. There are no known aetiologic factors other than the rare event of previous irradiation (13).
Clinical signs and symptoms may be inconspicuous, consisting of a low growing bony hard swelling of the jaw bone. Mobility of teeth only occurs in a late stage. In case of mandibular involvement the first sign of malignany may be paraesthesia or anaesthesia of the ipsilateral site of the lower lip. The radiographic appearance may vary from diffuse radiolucent to densely opaque. Radiographic features suggestive of osteosarcoma are irregular widening of the periodontal ligament and loss of decortication of the follicular crypts of embedded teeth (Fig. 4).
Histopathologically, osteosarcoma may present in a wide range of morphologic variations and may mimic, amongst others, fibro-osseous lesions, giant cell granuloma, cementoblastoma, and osteoblastoma. Conventional osteosarcoma is divided in three subtypes: 1) osteoblastic, 2) chondroblastic, and 3) fibroblastic type (9).
Treatment consists of (neo)adjuvant chemotherapy and surgery (14). There is hardly a role for postoperative radiotherapy. If radically excised, the prognosis of osteosarcoma of the jaws is rather favourable. If metastases occur, the lungs are the most commonly affected organs.
Non-Hodgkins lymphoma
The oral cavity may be the primary site of non-Hodgkins lymphoma. The estimated annual incidence is 1:1.000.000 population. There is no gender preference. Non-Hodgkins lymphoma usually affects patients above their sixth decade.
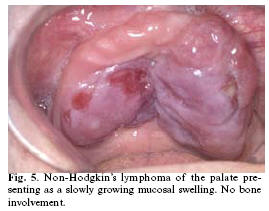
There are no known aetiologic factors for non-Hodgkins lymphoma, except the rare event of an underlying human immunodeficiency virus infection. All sites of the oral cavity may be affected, but the palate seems to be a site of preference. The clinical presentation may vary considerably with involvement of either the soft tissues or the bone. Non-Hodgkins lymphomas are occasionally very fast growing tumors but they also may present in a rather indolent way (15). Ulceration of the overlying epithelium may or may not be present (Fig. 5).
A biopsy is required to arrive at a proper diagnosis. Lymphoid tissue should be handled with care, since mechanic forces easily damage the cellular morphology, making the assessment of a reliable histopathological diagnosis difficult if not impossible. Most of the oral non-Hodgkins lymphomas are of the B-cell type (Table 2) (16). Before treatment is instituted patients will be staged according to the Ann-Arbor-system (Table 3) (17). In a group of 40 patients with an oral non-Hodgkins lymphoma, in whom the oral lesion was the presenting manifestation, 24 were staged as stage I, 12 as stage IV and 1 and 2 as stage II and III, respectively (15). Treatment consists usually of chemotherapy. The prognostic parameters are listed in table 4 (18).
Oral metastases
Approximately 1% of all oral cancers consist of metastases derived from tumors located elsewhere in the body. In one-third of the patients the oral lesion is diagnosed before the primary (19).
The primary is located in the common tumor sites, such as lung, breast, prostate and kidney. Oral metastases may either occur in the soft tissues or in the bone. In soft tissue involvement ulceration may or may not be present (Fig. 6)
Tumor markers may be of help in identifying the primary site. A well-known example is the use of prostate specific antigen in case of a metastasis of a prostate cancer.
In general, treatment possibilities of oral metastases are limited because of the often presence of multiple metastases.
Conclusions
The oral non-squamous malignant tumors form a heterogeneous group of tumors of unknown aetiology. In contrast to oral squamous cell carcinoma these non-squamous cell oral cancers are not preventable.
Probably due to their rare occurrence the signs and symptoms are often not recognized at an early stage. The histopathologic diagnosis may at times be difficult to establish with certainty. For the majority of these malignancies, non-Hodgkins lymphomas and oral metastases excepted, surgery is the treatment of choice, whereas the value of postoperative irradiation is questionable. Prognosis mainly depends on clinical stage and histopathologic type.
References
1. Ellis GL, Auclair PL. Tumors of the Salivary Glands. Atlas of Tumor Pathology, 4th Series, Fascicle 9. 1 ed. Washington, D. C.: Armed Forces Institute of Pathology; 2007. [ Links ]
2. Harris NL. Lymphoid proliferations of the salivary glands. Am J Clin Pathol. 1999 Jan;111(1 Suppl 1):S94-103. [ Links ]
3. Klussmann JP, Wagner M, Guntinas-Lichius O, Muller A. Detection of HHV-8 sequences and antigens in a Malt lymphoma associated with Sjogrens syndrome. J Oral Pathol Med. 2003 Apr;32(4):243-5. [ Links ]
4. Hew WS, Carey FA, Kernohan NM, Heppleston AD, Jackson R, Jarrett RF. Primary T cell lymphoma of salivary gland: a report of a case and review of the literature. J Clin Pathol. 2002 Jan;55(1):61-3. [ Links ]
5. Harris NL, Isaacson PG. What are the criteria for distinguishing Malt from non-Malt lymphoma at extranodal sites. Am J Clin Pathol. 1999 Jan;111(1 Suppl 1):S126-32. [ Links ]
6. Murga Penas EM, Hinz K, Röser K, Copie-Bergman C, Wlodarska I, Marynen P, Hagemeijer A, Gaulard P, Löning T, Hossfeld DK, Dierlamm J. Translocations t(11;18)(q21;q21) and t(14;18)(q32;q21) are the main chromosomal abnormalities involving Mlt/Malt1 in Malt lymphomas. Leukemia. 2003 Nov;17(11):2225-9. [ Links ]
7. Pavlidis NA, Drosos AA, Papadimitriou C, Talal N, Moutsopoulos HM. Lymphoma in Sjogrens syndrome. Med Pediatr Oncol. 1992;20(4):279-83. [ Links ]
8. Bahler DW, Swerdlow SH. Clonal salivary gland infiltrates associated with myoepithelial sialadenitis (Sjögrens syndrome) begin as nonmalignant antigen-selected expansions. Blood. 1998 Mar 15;91(6):1864-72. [ Links ]
9. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. European Study Group on Classification Criteria for Sjogrens Syndrome. Classification criteria for Sjogrens syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002 Jun;61(6):554-8. [ Links ]
10. Fox RI, Tornwall J, Michelson P. Current issues in the diagnosis and treatment of Sjogrens syndrome. Curr Opin Rheumatol. 1999 Sep;11(5):364-71. [ Links ]
11. McArthur CP, Subtil-DeOliveira A, Palmer D, Fiorella RM, Gustafson S, Tira D, Miranda RN. Characteristics of salivary diffuse infiltrative lymphocytosis syndrome in West Africa. Arch Pathol Lab Med. 2000 Dec;124(12):1773-9. [ Links ]
12. Craven DE, Duncan RA, Stram JR, OHara CJ, Steger KA, Jhamb K, Hirschhorn LR. Response of lymphoepithelial parotid cysts to antiretroviral treatment in HIV-infected adults. Ann Intern Med. 1998 Mar 15;128(6):455-9. [ Links ]
13. Chetty R. HIV-associated lymphoepithelial cysts and lesions: morphological and immunohistochemical study of the lymphoid cells. Histopathology. 1998 Sep;33(3):222-9. [ Links ]
14. Chan JK. Kuttner tumor (chronic sclerosing sialadenitis) of the submandibular gland: an underrecognized entity. Adv Anat Pathol. 1998 Jul;5(4):239-51. [ Links ]
15. Isacsson G, Isberg A, Haverling M, Lundquist PG. Salivary calculi and chronic sialoadenitis of the submandibular gland: a radiographic and histologic study. Oral Surg Oral Med Oral Pathol. 1984 Nov;58(5):622-7. [ Links ]
16. Tiemann M, Teymoortash A, Schrader C, Werner JA, Parwaresch R, Seifert G, Kloppel G. Chronic sclerosing sialadenitis of the submandibular gland is mainly due to a T lymphocyte immune reaction. Mod Pathol. 2002 Aug;15(8):845-52. [ Links ]
17. Capaccio P, Ottaviani F, Manzo R, Schindler A, Cesana B. Extracorporeal lithotripsy for salivary calculi: a long-term clinical experience. Laryngoscope. 2004 Jun;114(6):1069-73. [ Links ]
18. Sheen TS, Tsai CC, Ko JY, Chang YL, Hsu MM. Undifferentiated carcinoma of the major salivary glands. Cancer. 1997 Aug 1;80(3):357-63. [ Links ]
19. Merrick Y, Albeck H, Nielsen NH, Hansen HS. Familial clustering of salivary gland carcinoma in Greenland. Cancer. 1986 May 15;57(10):2097-102. [ Links ]
20. Jen KY, Cheng J, Li J, Wu L, Li Y, Yu S, et al. Mutational events in LMP1 gene of Epstein-Barr virus in salivary gland lymphoepithelial carcinomas. Int J Cancer. 2003 Jul;105(5):654-60. [ Links ]
21. Pinkston JA, Cole P. Incidence rates of salivary gland tumors: results from a population-based study. Otolaryngol Head Neck Surg. 1999 Jun;120(6):834-40. [ Links ]
22. Auclair PL. Tumor-associated lymphoid proliferation in the parotid gland. A potential diagnostic pitfall. Oral Surg Oral Med Oral Pathol. 1994 Jan;77(1):19-26. [ Links ]
23. Maiorano E, Lo Muzio L, Favia G, Piattelli A. Warthins tumour: a study of 78 cases with emphasis on bilaterality, multifocality and association with other malignancies. Oral Oncol. 2002 Jan;38(1):35-40. [ Links ]
24. Yu GY, Liu XB, Li ZL, Peng X. Smoking and the development of Warthins tumour of the parotid gland. Br J Oral Maxillofac Surg. 1998 Jun;36(3):183-5. [ Links ]
25. Miyake H, Matsumoto A, Hori Y, Takeoka H, Kiyosue H, et al.Warthins tumor of parotid gland on Tc-99m pertechnetate scintigraphy with lemon juice stimulation: Tc-99m uptake, size, and pathologic correlation. Eur Radiol. 2001;11(12):2472-8. Epub 2001 Mar 17. [ Links ]
26. Gunduz M, Yamanaka N, Hotomi M, Kuki K, Yokoyama M, Nakamine H. Squamous cell carcinoma arising in a Warthins tumor. Auris Nasus Larynx. 1999 Jul;26(3):355-60. [ Links ]
27. Medeiros LJ, Rizzi R, Lardelli P, Jaffe ES. Malignant lymphoma involving a Warthins tumor: a case with immunophenotypic and gene rearrangement analysis. Hum Pathol. 1990 Sep;21(9):974-7. [ Links ]
28. Takezawa K, Jackson C, Gnepp DR, King TC. Molecular characterization of Warthin tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998 May;85(5):569-75. [ Links ]
29. Lewis PD, Baxter P, Paul Griffiths A, Parry JM, Skibinski DO. Detection of damage to the mitochondrial genome in the oncocytic cells of Warthins tumour. J Pathol. 2000 Jul;191(3):274-81. [ Links ]
30. Arida M, Barnes EL, Hunt JL. Molecular assessment of allelic loss in Warthin tumors. Mod Pathol. 2005 Jul;18(7):964-8. [ Links ]
![]() Correspondence:
Correspondence:
I. van der Waal, DDS, PhD
VU university medical centre (VUmc)/
Academic Centre for Dentistry Amsterdam (ACTA)
P.O. Box 7057
1007 MB Amsterdam
The Netherlands
E-mail: i.vanderwaal@vumc.nl
Received: 20-05-2007
Accepted: 10-06-2007