INTRODUCTION
Atherosclerotic cardiovascular disease (ASCVD) continues to be the leading cause of death in the U.S.1 The risk of ASCVD increases with age, thus older adults assume the greatest burden of ASCVD risk.2 While high intensity statin therapy (HIST) is the gold standard therapy for decreasing the risk of ASCVD, there is significant debate surrounding the use of HIST in older adults with ASCVD due to lack of high-quality, randomized controlled trial (RCT) evidence of its effectiveness.3,4 While the 2013 American College of Cardiology/American Heart Association Task Force (ACC/AHA) guideline on the treatment of blood cholesterol to reduce ASCVD risk in adults does recommend moderate intensity statin therapy (MIST) for patients >75 years of age with clinical ASCVD, the guideline states that there is not enough information to clearly support HIST use in this patient population.5,6
Subgroup analyses of older patients have identified a cardiovascular benefit with statin therapy in older patients. For example, the Cholesterol Treatment Trialist’s Collaboration Study, using data from 26 RCT, identified that more intensive statin regimens produced further reduction in major vascular events and a similar preventive benefit of statin therapy across all age groups.7 In addition, a sub-group analysis of Veterans Affairs’ patients between 76 to 84 years of age reported significantly lower annual mortality rates in the HIST compared to the MIST groups.8
While there is conflicting evidence of an increased protective benefit of HIST in older patients with ASCVD, minimal “real-world” data regarding the use of HIST in patients >75 years with validated ASCVD exist. One cross-sectional study examined HIST use between patients with validated ASCVD >75 and ≤75 years and reported that those >75 years were significantly less likely to receive HIST (23.5% vs. 36.2%, p<0.001).9 Using claims data to identify patients with unvalidated cardiovascular disease who were >74 years, another cross-sectional study reported that 17.1% of females and 15.1% of males received HIST.10
Kaiser Permanente Colorado (KPCO), an integrated health care delivery system providing care to more than 660,000 patients in Colorado at 30 medical offices has a comprehensive cardiac risk reduction service called the Clinical Pharmacy Cardiac Risk Service (CPCRS). The CPCRS is a clinical pharmacy specialist-managed, physician-directed, protocol-driven secondary cardiovascular prevention service that uses a systems-based approach to focus on the long-term medication management of more than 16,000 patients with ASCVD.11-13 Greater than 95% of KPCO members with ASCVD are enrolled in the CPCRS. Clinical pharmacy specialists review patients enrolled in CPCRS and establish treatment goals collaboratively with physicians. Patients enrolled in CPCRS are managed under collaborative drug therapy management (CDTM) protocols, with each patient being offered all available evidence-based therapies in attempts to attain optimal patient outcomes. The CDTM protocols do not discriminate treatment recommendations based on patient age, thus the decision to use HIST is based on shared-decision making between the clinical pharmacy specialist, patient and physician.
The purpose of this study was to describe the trends over time and identify patient characteristics associated with the application of HIST among patients >75 years of age with validated ASCVD.
METHODS
Study design and setting
This was a retrospective, cross-sequential study of intensity of statin use in older patients with validated ASCVD and enrolled in the CPCRS at KPCO. Kaiser Permanente Colorado utilizes an electronic medical record (EMR) that provides e-prescribing capabilities. In addition, all 30 Denver/Boulder KPCO medical offices have a pharmacy and KPCO provides mail-order services where members are dispensed subsidized prescription medications. Information on prescriptions dispensed from these pharmacies are maintained in KPCO administrative databases. Coded and free-text medical, laboratory, emergency department, hospitalization, and membership information from within the delivery system, as well as from other contracted and affiliated facilities, are captured in KPCO’s administrative and claims databases. The KPCO institutional review board reviewed and approved all study activities with a waiver of informed consent.
Study population and procedures
Patients with ASCVD, defined as history of acute myocardial infarction (AMI), coronary artery bypass (CABG), percutaneous coronary intervention with/without stent (PCI) and enrolled in CPCRS were included. Queries of KPCO administrative databases were used to collect data from January 1, 2007 to December 31, 2016. Each calendar year was divided into halves (calendar-half) defined as January 1 to June 30 for the first half and July 1 to December 31 for the second half. The index date was January 1 for the first half of the year and July 1st for the second half of the year. Patients had to be 76 years or older as of the index date to be included in statin intensity assessment for the calendar-half. In addition to being 76 years or older, patients had to have been enrolled in the KPCO CPCRS.11 Statin intensity level was assessed during each calendar-half of the study time period. Patients could be included in multiple calendar-halves if they met criteria. Patients who had a statin prescription ordered to a non-KPCO pharmacy at any time during the study period were excluded as the accuracy of their dispensing history could not be verified. Patients who received hospice care at any time during the respective calendar-half or died during the 90 days after the index date of the respective calendar-half were also excluded. Patients were categorized as receiving statin therapy if they had a statin dispensed at any time during calendar-half under study. Statin intensity level was determined with the first statin dispensing during each calendar-half year.
Study outcomes
The primary outcome was the trend in rates of statin intensity over a 10-year timeframe in patients with ASCVD who were 76 years or older. Patients were categorized as having received no (NIST), low (LIST), MIST, and HIST during each calendar-half that they were eligible. Trends in intensity level are reported overall and individually by female and male patients. For patients with multiple statin dispensing dates during the respective calendar-half, the statin dispensed on the date most proximal to the index date was used to determine statin intensity level. The second objective was to identify patient characteristics associated with HIST use in patients with ASCVD who were 76 years or older. Patients who had a HIST dispensing in any calendar-half they were eligible were considered a HIST patient while patients who had MIST, LIST, or NIST for all calendar-halves that they were eligible were considered a Non-HIST patient.
Data collection
Information on dispensed prescription medications was obtained from queries of the KPCO electronic Prescription Information Management database using Generic Product Identifier Codes. Information on statins ordered for non-KPCO pharmacies was obtained from the EMR. Information on patient characteristics, including age, membership, and CPCRS enrollment, was obtained from queries of KPCO administrative databases. Patient characteristics were assessed during the six months prior to the index date of the first calendar-half that the patient was categorized as a HIST or Non-HIST patient. Characteristics included information on age, sex, race, Hispanic ethnicity, cardiovascular disease type, comorbidities, non-statin medication dispensings, and socioeconomic status.
Data analysis
No a priori power analysis was performed as this study was primarily descriptive in nature and all patients meeting eligibility criteria during the study period were included. Age was determined as of the index date for each calendar-half. Patients were categorized as HIST, MIST, LSIT, or NIST for each calendar-half and as HIST and Non-HIST overall. Daily doses were calculated using dispensed statin information (drug name, drug strength, quantity dispensed, days of drug supplied) and then categorized by intensity level (online Appendix).5 Index cardiovascular diseases were categorized as AMI, AMI + cardiac intervention, and cardiac intervention only. Interventions included CABG and PCI. Tobacco use included cigarette, pipe, chew, snuff, and vapor use.
A chronic disease score (CDS), a validated measure of the burden of chronic illness, was calculated for each patient using ambulatory prescription medication dispensings.14 The CDS ranges in values from 0 to 36 with increasing values indicating a higher burden of chronic illness. The presence of specific comorbidities was determined using the Quan adaptation of the Charlson comorbidity index (CCI).15 The algorithm was applied to diagnoses to provide a 30-point comorbidity score for each patient.
The percentages of patients in each intensity level was determined by summing the total count of patients per intensity level per calendar-half and dividing this value by the total count of patients eligible for inclusion in the calendar-half. Percentages are reported overall and separately by females and males. Percentages were graphed to illustrate trends in intensity level over a 10-year period. The Cochran-Armitage test was used to assess for linear trends, overall, in statin intensity over time.
Patient characteristics are reported as means, medians, and standard deviations for interval- and ratio-level variables (e.g., age) and percentages for nominal- and ordinal-level data (e.g., sex, co-morbidity history). Comparisons between the HIST and Non-HIST groups were made with parameteric/non-parametric t-tests, as applicable, for interval- and ratio-level data and chi-square tests of association for nominal- and ordinal-level data. An adjusted logistic regression model was constructed with HIST use as the dependent variable and patient characteristics as the independent variable to determine factors independently associated with HIST use. Characteristics included in the model (age, sex, CDS, CCI, race, Hispanic ethnicity, tobacco use, cardiovascular disease type, congestive heart failure (CHF), peripheral vascular disease (PVD), diabetes, renal disease, hypertension, and depression comorbidities, anti-platelet, angiotensin II receptor blocker, angiotensin converting enzyme inhibitor, and beta-blocker dispensing, and over-the-counter (OTC) aspirin use) were determined based on clinical judgement and univariate analysis. The alpha was set at 0.05.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All aspects of the study were reviewed and approved by the KPCO Institutional Review Board.
RESULTS
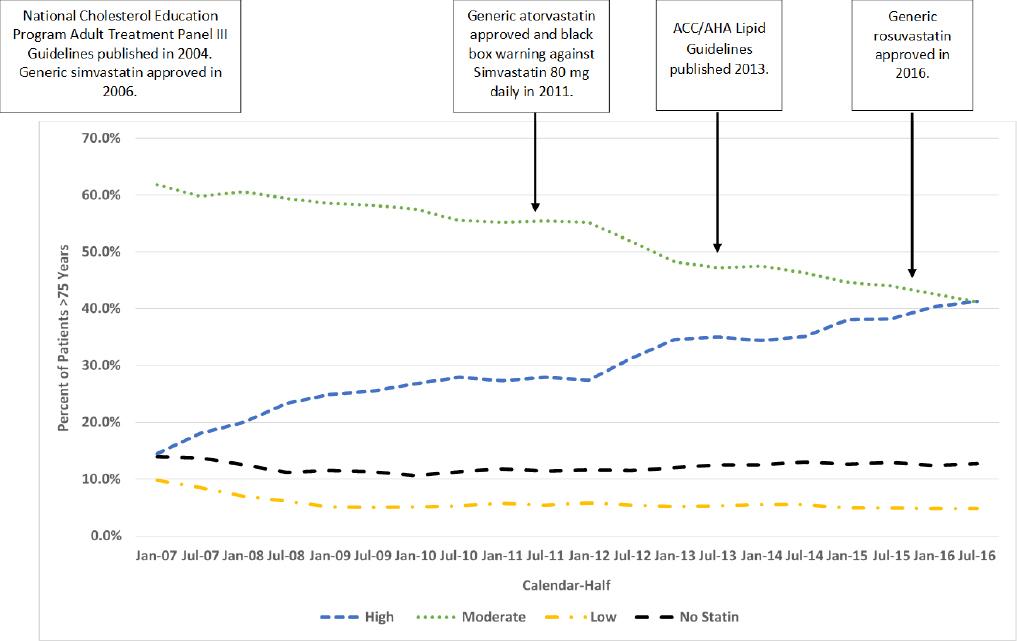
A total of 5,453 patients were included. Patients, overall, had a mean age of 79.8 years, were primarily male, white, and non-Hispanic, had a high burden of chronic disease, and their cardiovascular disease was identified from a cardiac intervention (Table 1). Approximately 2% of patients were excluded for using non-KPCO pharmacies. Overall, the percentage of patients who received HIST increased from 14.5% as of January 1, 2007 to 41.3% as July 1, 2016 (p<0.001 for trend) (Figure 1). Conversely, the percentage of patients who received MIST and LIST decreased from 61.8% and 9.8% as of January 1, 2007 to 41.2% and 4.8% as July 1, 2016, respectively (both p<0.001 for trend). The percentage of patients who received no statin fluctuated but remained in the 13-14% range (p>0.05 for trend). There were 801 (14.7%) patients who were titrated from a lower intensity statin to HIST during the study period.
Table 1. Baseline Characteristics by Statin Intensity Status (N=5453)
| Characteristic | High Intensity Statin Use (n=2119) | No, Low, Moderate Intensity Statin Use (n=3334) | p-value |
|---|---|---|---|
| Mean Agea(years, SD) | 78.5 (3.4) | 80.7 (5.0) | <0.001 |
| Female (n, %) | 766, 36.2% | 1411, 42.3% | <0.001 |
| White Race (n, %) | 1803, 85.1% | 2650, 79.5% | <0.001 |
| Hispanic Ethnicity (n, %) | 153, 7.2% | 228, 6.8% | 0.590 |
| Cardiovascular Disease Type (n, %) | |||
| AMI Only | 349, 16.5% | 827, 24.8% | <0.001 |
| AMI + Interventionb | 670, 31.6% | 1023, 30.7% | 0.467 |
| Interventionb Only | 1100, 51.9% | 1484, 44.5% | <0.001 |
| Tobacco Use (n, %) | 268, 12.7% | 380, 11.4% | 0.165 |
| Comorbidity Diagnosisc (n, %) | |||
| Cerebrovascular Disease | 212, 10.0% | 381, 11.4% | 0.100 |
| Depression | 242, 11.4% | 335, 10.1% | 0.108 |
| Diabetes Mellitus | 623, 29.4% | 843, 25.3% | <0.001 |
| Heart Failure | 395, 18.6% | 705, 21.2% | 0.025 |
| Hypertension | 1321, 62.3% | 2030, 60.9% | 0.283 |
| Pulmonary Disease | 495, 23.4% | 845, 25.3% | 0.097 |
| Peripheral Vascular Disease | 387, 18.3% | 483, 14.5% | <0.001 |
| Renal Disease | 600, 28.3% | 834, 25.0% | 0.007 |
| Rheumatologic Disease | 47, 2.2% | 105, 3.2% | 0.041 |
| Medications (n, %) | |||
| Anti-Plateletd | 580, 27.4% | 728, 21.8% | <0.001 |
| Angiotensin II Receptor Blockerd | 344, 16.2% | 477, 14.3% | 0.052 |
| Angiotensin Converting Enzyme Inhibitord | 931, 43.9% | 1441, 43.2% | 0.604 |
| Beta-Blockerd | 1645, 77.6% | 2403, 72.1% | <0.001 |
| OTC Aspirin | 2035, 96.0% | 3083, 92.5% | <0.001 |
| Mean Chronic Disease Scored (SD) | 6.0 (2.9) | 6.1 (3.1) | 0.001 |
| Mean Charlson Comorbidity Indexc (SD) | 2.4 (2.3) | 2.3 (2.3) | 0.282 |
| Mean Family Income (SD) | USD 64303 (USD 22511) | USD 61880 (USD 22283) | <0.001 |
| Mean Percent of Household with at Least Some College Education (SD) | 64.5% (18.3) | 62.9% (18.5) | 0.003 |
a-As of index date
b-Interventions include coronary artery bypass grafts and percutaneous coronary interventions
c-From diagnoses recorded during the 180 days prior to index date
d-From prescription medication dispensings during the 180 days prior to index date
AMI- acute myocardial infarction, SD - standard deviation
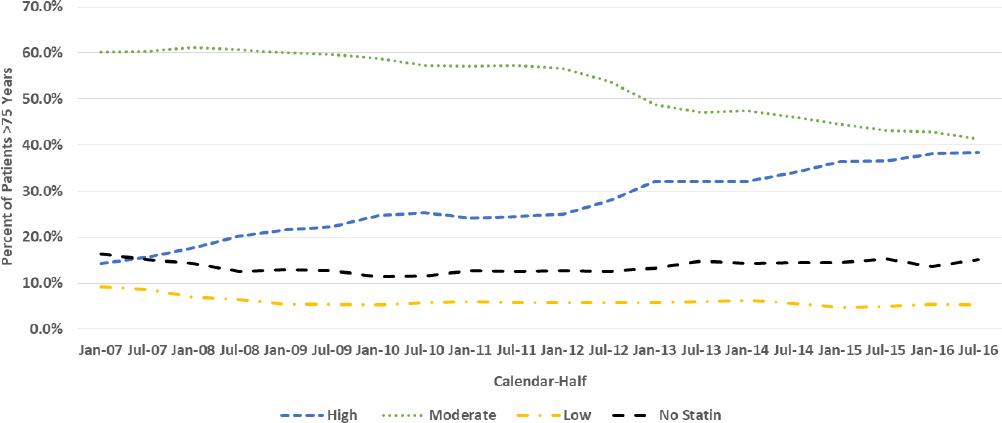
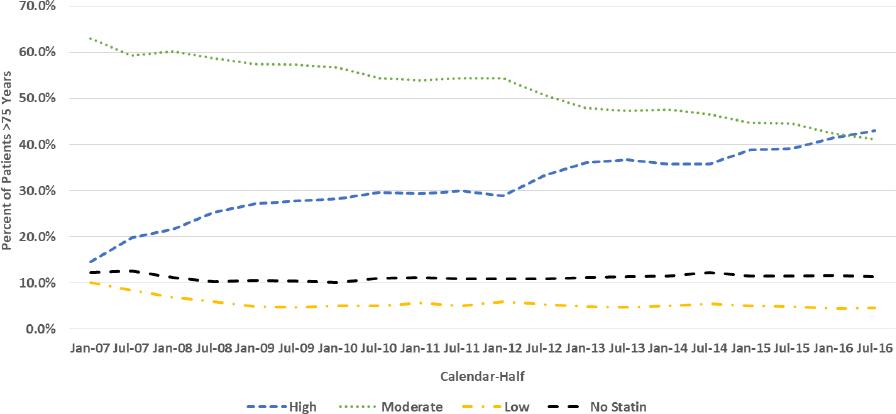
Similar trend patterns were seen for females (Figure 2) and males (Figure 3). The percentage of females and males who received HIST increased from 14.3% and 14.6% as of January 1, 2007 to 38.5% and 43.0% as July 1, 2016, respectively, while MIST use decreased from 60.2% and 63.0% as of January 1, 2007 to 41.3% and 41.1% as July 1, 2016, respectively. The percentage of female and male patients who received LIST decreased approximately by half over the study period while the percentages who received no statin fluctuated over a narrow range.
Overall, a total of 2,119 (38.9%) and 3,334 (61.1%) patients were categorized as HIST and Non-HIST, respectively. In univariate analysis, HIST patients had lower mean age and CDS with higher mean family income and percent of household with some college education (all p<0.05) (Table 1). HIST patients were more likely to be male and white, have had only a cardiac intervention, have PVD, diabetes, and renal disease, had a dispensing of anti-platelet and beta-blocker medication, and had OTC aspirin use (all p<0.05). Non-HIST patients were more likely to have had an AMI only and have CHF and a rheumatologic disease.
In multivariable analysis, factors independently associated with having received HIST include white race (adjusted odds ratio (aOR)=1.51), depression (aOR=1.28), PVD (aOR=1.32), and renal disease (aOR=1.27) comorbidities, anti-platelet (aOR=1.42) and beta-blocker (aOR=1.37) dispensings, and OTC aspirin use (aOR=1.52) (Table 2). Factors associated with being less likely to having received HIST include age (aOR=0.89), female sex (aOR=0.88), CDS (aOR=0.96), and AMI only (aOR=0.71) and AMI + intervention (aOR=0.88) compared to intervention only.
Table 2. Factors Associated with High Intensity Statin Use
| Factor | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Age | 0.89 | 0.88 - 0.91 |
| Female Sex | 0.88 | 0.78 - 0.99 |
| Charlson Comorbidity Index | 0.99 | 0.94 - 1.04 |
| Chronic Disease Score | 0.96 | 0.94 - 0.99 |
| White Race | 1.51 | 1.26 - 1.81 |
| Hispanic Ethnicity | 1.25 | 0.96 - 1.63 |
| Cardiovascular Disease Type(n, %) | ||
| AMI Only | 0.71 | 0.60 - 0.83 |
| AMI + Intervention | 0.88 | 0.77 - 0.99 |
| Intervention Only | --- | ----- |
| Tobacco Use (n, %) | 0.97 | 0.81 - 1.57 |
| Comorbidity Diagnosis (n, %) | ||
| Cerebrovascular Disease | 0.86 | 0.70 - 1.05 |
| Depression | 1.28 | 1.05 - 1.54 |
| Diabetes Mellitus | 1.15 | 0.99 - 1.35 |
| Heart Failure | 0.99 | 0.83 - 1.17 |
| Hypertension | 1.03 | 0.90 - 1.17 |
| Pulmonary Disease | 0.94 | 0.80 - 1.10 |
| Peripheral Vascular Disease | 1.32 | 1.11 - 1.57 |
| Renal Disease | 1.27 | 1.06 - 1.52 |
| Rheumatologic Disease | 0.73 | 0.50 - 1.05 |
| Medications (n, %) | ||
| Anti-Platelet | 1.42 | 1.24 - 1.63 |
| Angiotensin II Receptor Blocker | 1.11 | 0.93 - 1.32 |
| Angiotensin Converting Enzyme Inhibitor | 1.07 | 0.93 - 1.23 |
| Beta-Blocker | 1.37 | 1.19 - 1.57 |
| OTC Aspirin | 1.52 | 1.16 - 1.99 |
AMI- acute myocardial infarction
DISCUSSION
This retrospective trend analysis of over 5,400 patients with ASCVD who were 76 years and older identified that the percentage of patients who received HIST increased by approximately 1.9 times over a 10-year study period. This increase was offset by decreases in MIST and LIST as the percentages of patients who received MIST and LIST decreased by approximately one third and half, respectively, over the study period. In contrast, the percentage of patients who received no statin fluctuated slightly over the study period. These trends in statin intensity were similar for both females and males. To our knowledge, this is the first study to examine 10-year trend in HIST use in female and male patients with validated ASCVD who were 76 years and older. Our findings are important since they provide information from a large cohort of a patient population that has been underrepresented in analyses of statin intensity for secondary prevention and suggest that practitioners are adjusting and initiating statin dosing to the highest intensities despite guideline ambivalence of HIST effectiveness.
Few other studies have assessed HIST use in older patients with ASCVD. The PALM registry study assessed a cross-sectional subset of patients with ASCVD (n=1038) who were >75 years and identified that 23.5% of patients in the study received HIST as of 2015.9 The investigators did not perform analysis of their sample by patient sex. Our finding of 38.0% HIST use, overall, as of January 1, 2015 is numerically a considerably higher percent of patients who received HIST. Our finding may be different since we only included patients with coronary ASCVD (i.e., AMI, CABG, PCI with or without stent) while PALM included a broader range of patients with clinical ASCVD. In addition, the PALM patients were from diverse clinics (potentially with and without DSM services) while ours were patients managed by a clinical pharmacy cardiac risk service.9 Rosenson and colleagues assessed statin intensity with claims data among patients >75 years 30 days after hospital discharge with a myocardial infarction diagnosis.16 They reported that from 2011 through 2014, the percentage of patients who had a HIST prescription dispensed increased from 19.2% to 47.4%.16 During a similar time period, we observed an increase in HIST dispensings from 27.3% to 35.1%. Our findings may have differed since Rosenson and colleagues only assessed patients within 30 days after hospital discharge for an acute MI and excluded patients who received simvastatin.16 A recent study of CPCRS patients with ASCVD evaluated trends in HIST use in younger patients (i.e., 21-75 years old). Similar trends in HIST use were identified with HIST use increasing from 44% in 2007 to 67% in 2016 (p< 0.001 for trend).17
There are potential elements that may have influenced the increase in HIST use we observed in our cohort. The fastest rate of increase in HIST use that we observed occurred prior to 2011 (2007 - 2010, 0.9x vs. 2011 - 2016, 0.5x). The increasing use of HIST during the earlier years of our study may have been driven by the CPCRS CDTM protocol that emphasized an LDL-Cholesterol (LDL-C) goal <70 mg/dL after the publication of the National Cholesterol Education Program Adult Treatment Panel III guidelines.18 In addition, these guidelines endorsed that older patients will benefit from therapeutic lowering of LDL-C.18 Furthermore, the availability of generic simvastatin in 2006 (immediately preceding our study period) provided practitioners with a statin that could be dosed easily at high intensity levels (e.g., 80 mg tablet once daily) at an affordable cost.19 During the latter years of our study, the availability of generic atorvastatin in late 2011 and rosuvastatin in early 2016 provided less expensive and more tolerable HIST while the black-box warning applied to simvastatin 80 mg in mid-2011 likely provided an impetus for increased use of atorvastatin.19 Furthermore, the release of the 2013 ACC/AHA Lipid Guidelines promoted changes to the CPCRS CDTM protocol to focus on reaching HIST and then assessing LDL-C goal.5 Combined, these elements likely drove increased HIST use.
Besides the temporal trend in increased use of HIST, we identified numerous factors that were associated independently with HIST (younger, male, and white patients and those with a lower burden of chronic disease, a dispensing of an anti-platelet or beta-blocker, and a depression, PVD, or renal disease comorbidity). Rosenson and colleagues identified that males and a beta-blocker or antiplatelet dispensing were associated with HIST.16 The PALM registry study reported that younger age was associated with HIST.9 A fascinating finding from our study was that patients with an AMI without an intervention were less likely to have received HIST than patients who underwent a cardiac intervention. We hypothesize that some of the AMI were not driven by atherosclerosis but takotsubo cardiomyopathy, vasospasm, or dissection; thus, HIST may not be appropriate therapy for such patients.
Another interesting yet reasonable finding was that patients with a higher burden of chronic illness were less likely to have received HIST. Such patients may have poorer prognoses and use of HIST may not have affected their outcomes. That patients with a depression comorbidity were more likely to have received HIST is a noteworthy finding; however, it may simply be related to these patients utilizing the health system more frequently, thus, increasing their chances of follow up with a clinical pharmacist or other lipid-focused practitioner. It is rational that patients with PVD were more likely to have received HIST since their PVD along with ASCVD were both indications for statin therapy. Finally, that patients with renal disease were more likely to be HIST is logical since these patients are at very high risk for cardiac-related morbidity and mortality.
While we identified that 41.4% of patients with coronary ASCVD 76 years and older had received HIST at the end of our study period, it is reasonable to expect that this rate could have been and currently might be higher. The CDTM protocol that the CPCRS practices under lists advanced age as a mitigating circumstance to alter treatment strategies based on statin tolerance and other patient specific factors.13 In practice, this may have manifested in patients with advanced age being treated less aggressively as patients with an intolerance were treated with a lower intensity statin or no statin. If positive evidence accumulates on HIST-related outcomes in patients with ASCVD older than 76 years, practice may modify and optimize statin therapy to HIST in these patients.
Limitations
Our study captured a large number of outpatient adults with validated coronary ASCVD 76 years and older. We used broad entry criteria to study a diverse population that included both incident and prevalent statin users. Nevertheless, our study did have limitations. We relied on pharmacy dispensing records to determine statin intensity. We excluded patients who filled statins at non-KPCO pharmacies as we were unable to pull administrative data from these pharmacies. We do not suspect this impacted our overall findings as the proportion excluded for this reason was low (2%). Patients may have received instructions from their practitioner (e.g., split tablet and take half tablet per day) that were not captured in the pharmacy database. To offset this potential bias, we included all CPCRS patients, whether they had a dispensing for statin therapy or not, to provide a more precise HIST rate. In patients with multiple statin prescriptions, we assessed the first statin dispensed during the calendar-half instead of choosing the highest intensity statin dispensed. We took this as a more conservative approach to HIST use. Patients with ASCVD who were not managed in a DSM were not included so there was no comparator group. In addition, our study was conducted in one integrated health system, thus, our findings may not be generalizable to other systems. As some of our findings were confirmatory of other studies’ findings, the importance of our findings is likely generalizable to other systems. We did not assess changes in LDL-C with use of HIST. We examined a limited number of factors in our multivariate analysis. Other factors such as adverse statin reactions, previous statin intolerability, or patient-specific factors (e.g., benefit design, low LDL) may have contributed to Non-HIST use.
CONCLUSIONS
This study identified that the percentage of patients with ASCVD 76 years and older who received HIST substantially increased from 2007 to 2016. This trend was identified in both females and males. Patient factors independently associated with HIST included younger age, male, and white patients and patients with a lower burden of chronic disease, a dispensing of an anti-platelet or beta-blocker, and a depression, PVD, or renal disease comorbidity. Future comparative effectiveness research should be conducted in this patient population to examine cardiac-related outcomes with HIST use as evidence to support HIST use in this patient population is lacking.