Introduction
Ionizing radiation, such as X-rays, damages cells because it has sufficient energy to break chemical bonds in and cause damage to vital cellular components, causing cell death, cell repair, or cancer. This damage can be caused directly to cellular components or indirectly through the formation of reactive oxygen species from water. The severity of radiation damage depends on both absorbed dose size and frequency of exposure. A large dose may kill, but repeated small doses can increase cancer risk.
On average, a person receives a radiation dose of 3 mSv/year from both artificial and natural sources, with radon gas and medical procedures such as X-ray imaging and radiotherapy among the most important sources. Recently, the use of these imaging and therapeutic procedures has increased. Although this trend is helpful, it also makes radiation hygiene for both medical personnel and patients an increasingly important consideration. Radiation hygiene may include preparation of technical guides using the ALARA principle (As Low As Reasonably Achievable), education of medical staff and supporting a culture of radioprotection, using newer technologies that maintain quality of treatment but decrease dose, and performing risk-benefit analysis of procedures that involve ionizing radiation1.
Although radiation hygiene is helpful in limiting exposure, it will be difficult to eliminate all exposure. Of particular concern are parts of the body that may be exposed during common procedures. Centrally located organs like the heart would have the greatest exposure. For example, 40% of all X-ray images taken worldwide involve the thorax2 giving the heart a dose each time. It also may receive collateral doses during radiotherapy treatments of thoracic tumors, which may have secondary effects. For instance, breast cancer radiotherapy carries the risk of cardiac death years later3. Although the heart is not considered highly sensitive to radiation, a dose of 40 Gy broken into a conventional radiotherapy regimen of 10 Gy/week can cause some myocardial degeneration. A dose greater than 60 Gy for the whole heart can cause death by pericardial effusion and constrictive pericarditis.
Given this potential for collateral damage in spite of good radiation hygiene, the possibility of improving the body’s resistance to and ability to recover from radiation doses is appealing. One promising area of research is supplementation with radioprotective foods and substances before, during, and after radiation dose. One such food, whole wheat, has been found to have cardioprotective properties4 most likely due to its antioxidant, nutritionally essential polyunsaturated fatty acids, and vitamin content. Found at highest concentrations in the germ, these components may improve cellular repair, decrease inflammation (5, improve electrical properties of the myocardium (6) and scavenge free radicals post-exposure (7,8,9,10.
Although the impact of diet on the development of resistance to radiation damage is often overlooked11, some research using gastric, esophageal, and hepatic rat tissue7,12, is promising in that animals fed with wheat germ better maintain cellular morphology post exposure to X-rays. Given the frequent exposure of the heart to concomitant radiation during medical procedures, here we aim to extend previous research by evaluating the radioprotective effects of a wheat germ-enriched diet on cardiac tissue from rats exposed to X-rays.
Methods
Animals and diet
The study took place in the animal facility and toxicology laboratory of the School of Pharmacy and Biochemistry of the National University of Trujillo (UNT). Animals were treated in accordance with international guidelines for handling of experimental animals. The study was carried out using two diets - wheat germ (WG) or the standard animal facility diet of corn and barley, which does not contain wheat (NW). Germ from a native variety of wheat (Triticum aestivum) was obtained on the open market in Otuzco province, Peru and was confirmed by the Herbarium Truxillensis of UNT.
Male rats Rattus rattus var. Albinus between 200 and 250 g were obtained from the Animal Facility of the National Health Institute, Peru. On day 1, the rats were assigned to be in one of 4 groups of 6 animals each: NW diet, no radiation exposure (NWNE); WG diet, no radiation exposure (WGNE); NW diet, radiation exposure (NWE); WG diet, radiation exposure (WGE). All animals were housed in individual cages and received 10 g of food daily. Water was provided ad libitum. Tiles were placed in the cages so that the animals had access to all of the food provided.
Radiation treatment of animals
On day 16, rats of groups NWE and WGE were immobilized with pentobarbital in saline (3 mg/kg). The rats were exposed to 18 mSv of X-rays in a standard X-ray imaging device at an ES-SALUD hospital at El Porvenir district, La Libertad Region, Peru. The animals continued with their diet for an additional 16 days after exposure.
All animals were sacrificed with a dose of 12 mg/kg pentobarbital in saline on day 32. The heart was extracted and preserved in 10% formaldehyde.
Results
The presence of radiation-damaged cells was observed in samples exposed to X-rays (Fig. 1C and Fig. 1D; Fig. 2C and 2D). However, the severity of tissue damage is clearly different: qualitatively comparing Fig 1D and Fig 2C makes it clear that the NWE group suffered more cardiac damage (necrosis, hyperimea and karyolysis) when compared to the WGE group which had less damage (no karyolysis, reduced necrosis and hyperemia).
In contrast, cellular viability, indicated by the presence of fusiform nucleii and proper celullar architect
ure13 was maintained in groups not exposed to radiation (compare Fig. 1A and B to Fig. 2A and B). Myocardiocytes, myofibrils, and connective tissue cells and nuclei have normal morphology. Taken together, it seems that the WG and NW diets maintain cardiac tissue viability in the absence of ionizing radiation, but the WG diet decreases the severity of radiation-induced cardiac damage.
Discussion
The use of ionizing radiation plays an important role in the medical sciences, such as in diagnostic imaging and radiotherapy. Although useful, ionizing radiation can cause tissue damage 14 by directly damaging vital cellular apparatuses or by generating reactive oxygen species (ROS) or other free radicals. Damage to DNA from these reactive species induces apoptosis, which includes an ordered fragmentation of internucleosomal DNA strands and condensation of nuclear chromatin, which causes disappearance of the nucleus15. These effects were observed in rat myocardium (Fig. 1C and D, Fig. 2C and D) exposed to a single 18 mSv dose from an imaging X-ray machine.
Of the two groups exposed to X-rays, the group fed wheat germ (WGE) experienced less myocardial damage than the group fed the standard diet (NWE). A likely explanation is that a diet of wheat germ provides some protection from the quantity of radiation-induced damage (Fig. 1D and Fig. 2C) in myocardium. A probable mechanism for this protection may be that wheat germ components, such as vitamins, organic acids, sterols, lipids, glutamic acid, proteins, antioxidants, and others16,17,18,19) may protect against ionizing radiation by directly absorbing it or by neutralizing or inhibiting the formation of free radicals20,21 as compared to the standard diet. This would leave fewer free radicals available to damage DNA. Wheat germ components may also stimulate cellular repair mechanisms. Additionally, antioxidants in wheat germ may be beneficial because they improve cardiac contraction through improved regulation of the flow of calcium ions13 maintaining myocardium during and after radiation exposure. Wheat germ contains lipids and sterols that maintain cell membranes that can also attenuate the effect of radiation damage, especially capillary membranes. This damage can initiate inflammatory processes that ultimately lead to heart disease22.
Although medical procedures involving ionizing radiation procedures have become safer due to improvements of radiation hygiene protocols, it is not possible to eliminate collateral exposure to ionizing radiation. Exposure of this type is of particular concern for organs like the heart because its central location frequently puts it in risk of exposure to ionizing radiation from medical imaging and radiotherapy of the thorax. Therefore, increasing the resistance of heart to radiation damage, such as through dietary supplementation23,24 is an appealing approach. The evidence that a wheat germ diet in rat can be radioprotective provides promise for additional dietary protocols to help build resistance to radiation damage.
Future studies that could complement and extend these results could include testing for biological markers of cell damage and cytogenetic assays25 among others.

Figure 1a: Representative micrographs of myocardium of rats fed the NW diet. (A) Micrograph of group NWNE myocardium at 400x. 1) Nucleus of cardiac muscular cells. 2) Fusiform nucleus of connective tissue cells. 3) Areas free of fibrils.

Figure 1b: Micrograph of group NWNE 100x showing branching and anastomosis of myocardium and vascularized connective tissue. 1) Fusiform nuclei of capillary endothelium. 2) Nucleus of cardiac muscular cells.
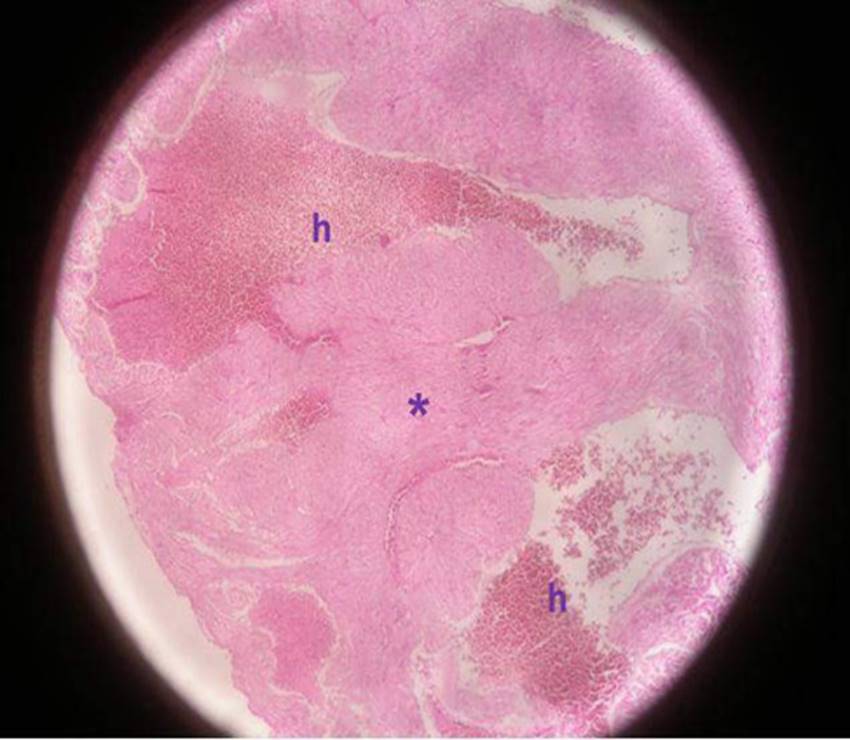
Figure 1c: Micrograph of the ventricular section of group NWE myocardium at 100 x showing marked hyperemia in blood vessels h) and loss of muscular architecture (*) with an absence of radial ordering. Ventricular section.
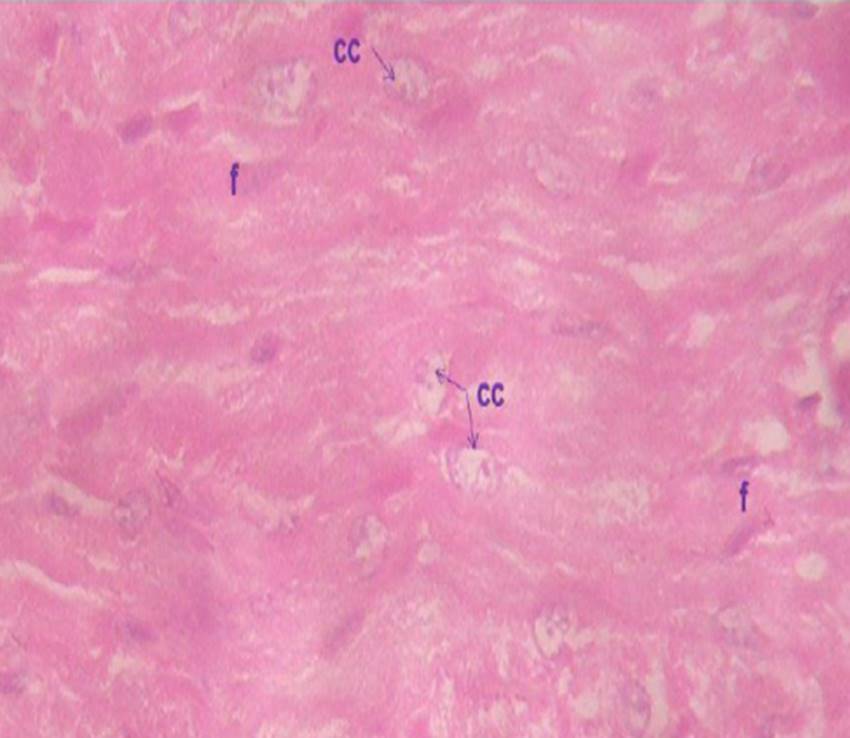
Figure 1d: Micrograph of the ventricular section of group NWE myocardium at 600 x, indicating the presence of anuclear cardiac cells, nucleii undergoing karyolisis cc), and f) Presence of fibrocytes in connective tissue. Ventricular section.

Figure 2a: Representative micrographs of myocardium of rats fed the WG diet. (A) and (B) Micrographs of group WGNE at 1000 x. (A) Marked staining of nucleii of cardiac muscle (m), fibrocytes (f), and endothelial cells (e) indicates tissue viability.

Figure 2b: Image indicates apparently normal pathology with branching and anastomosis of myocardium. Both muscular and fibrocyte cell nucleii are fusiform.

Figure 2c: (C) Micrograph of group WGE transversal cut at 400 x. A few anuclear muscle fibers indicate radiation-caused necrosis (*).

Figure 2d: Transversal cut (D) Micrograph of group WGE transversal cut at 1000 x. Presence of myocardial nucleii (*) and fibrocytes (arrow) indicate continued viability of the tissue with minimal radiation damage. Transversal cut
Study Limitation: A sham treatment group was not used.














