Highlights
World health organization identifies antimicrobial resistance as one of the most pressing challenges of future global health. World bank estimates that antimicrobial resistance could put an additional burden of one trillion US dollar on health care cost. As per the 2022 global antimicrobial resistance and use surveillance system (GLASS) report, MRSA has an alarming prevalence rate of 35%. MRSA is also one of the leading causes of nosocomial infections.
The development of complementary therapies using natural products is an attractive strategy to counter antimicrobial resistance. The combination of natural products with conventional antibiotics has a huge potential to address the problem of antimicrobial resistance. In addition, biofilm formation not only contributes to antimicrobial resistance but also enhances transmission, especially in the case of MRSA. Thus, developing antibiofilm agent also is an attractive strategy to tackle antimicrobial resistance and nosocomial infections. The activity of garlic and its organosulphur against MRSA is well established. However, studies on their use in combination of methicillin and β-lactam antibiotics are lacking. In this study we report that FGE sensitizes MRSA to methicillin and other β-lactam anitibiotics to which it was resistant. Further, FGE also inhibited biofilm formation of MRSA by inhibition surface binding. The study highlights potential of garlic in complementary therapy to treat MRSA and also as an antibiofilm agent to limit transmission.
Introduction
Staphylococcus aureus is generally a harmless commensal bacterium usually found on the skin and in the nose of healthy individuals. It can cause mild skin and soft tissue infections. It can also cause serious life-threatening infections such as bacteremia, pneumonia, endocarditis, and osteomyelitis if it enters the body or bloodstream. These infections are treated by penicillinase-resistant antibiotics such as flucloxacillin, cefazolin, etc. However, the emergence of MRSA, which is resistant to all β-lactam antibiotics, has severely complicated the treatment of S. aureus infections. Vancomycin is used to treat MRSA infections, but the emergence of vancomycin-resistant S. aureus is an acute healthcare problem1.
Nosocomial infections or healthcare-associated infections increase the morbidity and mortality of patients and impose a substantial economic burden on the healthcare system2. MRSA is one of the leading causes of nosocomial infections, according to Canters for disease control3. The contaminated hospital surfaces are crucial in transmitting nosocomial pathogens, including MRSA4. The ability of MRSA to survive on abiotic surfaces for extended time periods exacerbates the risk of infections in hospital settings5.
Biofilm is an aggregation of bacteria in a self-secreted extracellular polymeric substance formed on biotic and abiotic surfaces. Biofilm promotes the survival of bacteria by protecting them from harsh environmental conditions. MRSA can also form a biofilm enabling it to survive longer on hospital surfaces to cause infections6. The strategies to limit MRSA infections would be to contain its transmission by inhibiting MRSA biofilm formation and treating its infection with new or novel antibiotics.
Garlic (Allium sativum) is a prevalent food spice worldwide, especially in India, the Middle East, and Southeast Asia. Garlic consumption is associated with various health benefits such as anticancer, antidiabetic, immunomodulatory, antilipidemic, etc. Garlic also exhibits excellent antimicrobial activity and has been shown to inhibit a wide range of pathogenic bacteria, including drug-resistant strains. The health benefits, including the antibacterial property of garlic, are mainly due to its organosulphur compounds such as allicin, ajoene, diallyl sulphide (DAS), diallyl disulphide, diallyl trisulphide, etc7. The antibacterial activity of garlic and its organosulphur compounds against MSSA and MRSA is well studied8. However, limited studies have been performed on the antibiofilm potential of garlic and its compounds against MSSA and MRSA9. In a previous study, we reported the antibiofilm and antibacterial properties of FGE against multidrug-resistant Shiga toxin-producing Escherichia coli10. The principle antibacterial constituent of FGE is allicin, which decompose into various antibacterial allyl sulphides7. In this study, we investigate the antibiofilm property of FGE on MSSA and MRSA. We demonstrate the ability of sub-MIC concentrations of FGE and DAS to inhibit biofilm formation by MSSA and MRSA. Furthermore, the interaction of FGE with methicillin and other β-lactam antibiotics to inhibit MRSA is also examined.
Methods
Antibacterial activity assays
The antibacterial and antibiofilm properties of FGE were tested against three clinical isolates MSSA1-3 and MRSA1-3, by determining the MIC and zone of inhibition (ZOI) by microdilution and well diffusion assay respectively as described earlier10. The FGE was prepared by homogenizing garlic cloves and squeezing the juice using a muslin cloth. The freshly obtained garlic juice was centrifuged at 6000 rpm for 10 min, and the supernatant was collected as FGE, which was stored at -80 °C until further use. MIC was obtained by incubating 0.5 McFarland overnight cultures of isolates in Luria-Bertani (LB) media with various concentrations of FGE in 96 well plate for 24 h at 37 °C with shaking. The FGE was diluted tow-folds to give a concentration range from 375 µl/ml to 11.71 µl/ml. The ZOI was determined by spreading 0.5 MacFarland overnight cultures on LB agar plates with 5 mm wells which were filled with 5, 10, and 15 µl of FGE and methicillin (10 mcg) disc was used as antibiotic control and incubating at 37 °C for 24 h. The interaction of FGE with antibiotics was determined by ZOI experiments that were performed by combining FGE (4 μl which did not show any ZOI by itself) with antibiotics disc (penicillin-10 mcg, methicillin-10 mcg, cefoxitin- 30 mcg, cefepime- 30 mcg, and cefazolin- 30 mcg). Growth curve assay was performed by subculturing overnight MRSA culture at 37 °C with 180 rpm shaking in the presence of 1.5 % v/v FGE, 2.5 μg/ul methicillin, 1.5 % v/v FGE and 2.5 μg/μl methicillin, and no treatment as control. The growth was monitored by recording the absorbance at 600 nm at various time points.
Biofilm formation assay
Biofilm formation was quantified using crystal violet assay as described earlier10. The biofilm was allowed to form in 96 well plate, which was incubated with 0.5 MacFarland culture of the MSSA and MRSA for 72 h at 37 °C in the presence of 1, 2 and 4 % v/v FGE whereas untreated culture served as a control. Similarly, MSSA and MRSA biofilm was allowed to form in the presence of 0.1 and 0.5 % V/V of DAS, and untreated served as control. The biofilm was washed carefully thrice with 1X phosphate saline buffer (PBS) and air-dried overnight. The dried biofilm was stained with 0.1 % crystal violet solution for 10 min and washed twice with distilled water. The absorbed crystal violet was dissolved by adding 80 % ethanol, and absorbance was measured at 570 nm.
Scanning electron microscopy (SEM) analysis
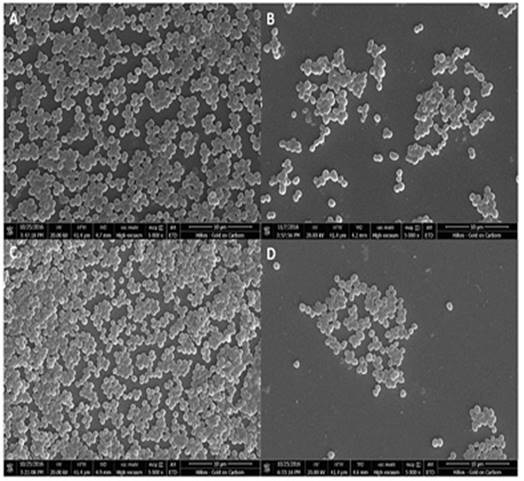
To capture SEM images, the biofilm was allowed to form on the coverslips placed in the wells of a 6 well plate which was incubated with 0.2 MacFarland bacterial cultures in the absence and presence of 2 % v/v FGE at 37 °C for 18 hrs. The coverslips with biofilm were carefully washed twice with 1XPBS and once with distilled water. The biofilm was fixed by treating with 0.25 % glutaraldehyde for 15 min, followed by dehydration by sequential ethanol treating (10 % to 90 %) for 10 min, and finally for 30 min in absolute ethanol. The biofilm was dried for 6 hrs at 130 °C in a hot air oven and gold sputter coated. SEM images were captured at an acceleration voltage of 20 kV using Nova Nano SEM 450.
Results
Antibacterial and antibiofilm activity of FGE
The activity of FGE against MSSA and MRSA was investigated by determining the MIC and ZOI. As expected, the MIC values shown in Table 1 indicate that FGE effectively inhibits the growth of both MSSA and MRSA strains. The ZOI values shown in Table 1 indicate that FGE inhibits the growth of not only MSSA but MRSA in a dose-dependent manner, as reported previously. The activity of FGE against MSSA and MRSA was comparable.
Table 1. Minimum inhibitory concentration and zone of inhibition values of FGE against MSSA and MRSA strains.
| Bacteria | MIC µl/ml | ZOI (mm) | ||
|---|---|---|---|---|
| 5 µl FGE | 10 µl FGE | 15 µl FGE | ||
| MSSA1 | 156.25±54.13 | 12.22±1.25 | 17.67±1.26 | 21.78±1.37 |
| MSSA2 | 250±108.25 | 11±2.83 | 17.22±1.87 | 21.11±1.31 |
| MSSA3 | 156.25±54.13 | 9±1.58 | 15.22±1.16 | 19.67±1.53 |
| MRSA1 | 187.5±0 | 10.56±0.56 | 15.33±0.33 | 19.44±0.68 |
| MRSA2 | 250±108.25 | 10.56±0.91 | 16.56±1.56 | 22.11±2.88 |
| MRSA3 | 312.5±108.25 | 14.11±2.84 | 18.11±2.95 | 22.44±3.13 |
MIC and ZOI reported as the average of triplicate experiments with SD. MIC=Minimum inhibitory concentration; ZOI=Zone of inhibition
After reconfirming the antibacterial activity of FGE against MSSA and MRSA, its ability to inhibit biofilm formation at sub-MIC concentration was investigated. It was interesting to see that sub-MIC concentrations of FGE significantly inhibited the biofilm formation of both MSSA and MRSA in a dose-dependent manner (Figure 1A). In the presence of 2 and 4 % v/v of FGE, the average biofilm inhibition of MSSA strains was 50 and 70 %, respectively. In the case of MRSA strains, the average inhibition was 49 and 78 % in the presence of 2 and 4 % v/v FGE, respectively. The results indicate that FGE significantly inhibits biofilm formation by MSSA and MRSA. To investigate if organosulphur compounds of garlic are responsible for the biofilm inhibition, the biofilm formation assay was performed in the presence of 0.1 % and 0.5 % V/V of DAS. The results shown in (Figure 1B), clearly indicated that 0.5 % V/V of DAS inhibited the biofilm formation of MSSA and MRSA by 33 % and 49 % respectively. The data suggest that organosulphur compounds are most likely responsible for inhibiting biofilm formation by MSSA and MRSA.
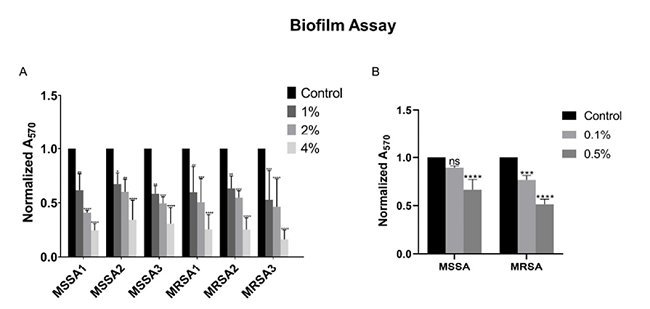
Figure 1. Biofilm formation assay. A) Inhibition of MSSA and MRSA biofilm formation at various concentrations of FGE as labelled. B) The inhibitory effect of different concentration of DAS on MSSA and MRSA biofilm formation as labelled. Untreated control sample was normalized to 1 and treated samples were compared with it. Error bars indicate that standard deviations from triplicates experiments and statistical significance between control and treatments was calculated by two-way ANOVA (*-P ≤ 0.05, **-P ≤ 0.01, ***- P ≤ 0.001, ****-P ≤ 0.0001).
SEM analysis of biofilm
To further confirm the inhibitory effects of FGE on biofilm formation SEM analysis was performed. The SEM images clearly show a significant reduction in the number of MRSA and MSSA attached to the coverslip in the presence of sub-MIC concentration of FGE (Figure 2). The results suggest that FGE significantly reduces biofilm formation by MSSA and MRSA by inhibiting the attachment of bacteria.
Interaction of FGE with methicillin and other β-lactam antibiotics
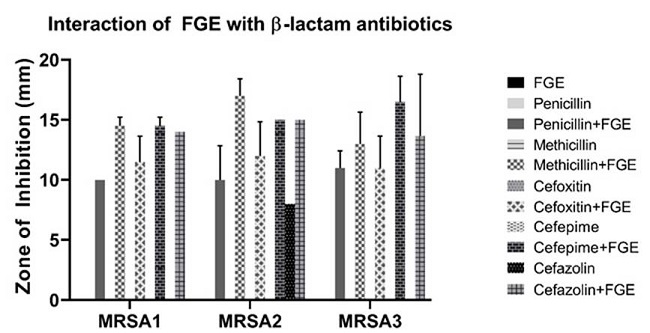
The interaction of FGE with methicillin was investigated by performing a growth curve assay. The growth curves are shown in (Figure 3); as expected there was no difference in the growth of control and 1.5 % v/v FGE, as this concentration is below MIC. The growth was slower in the presence of 2.5 μg/μl (very high concentration) of methicillin. It was interesting to see that the combination 2.5 μg/μl of methicillin and 1.5 % v/v FGE completely inhibited the growth of MRSA. This indicates that FGE was able to sensitive MRSA towards methicillin. To further investigate the interaction of FGE with other β-lactam antibiotics, ZOI experiments were performed by combining FGE (4 μl which did not show any ZOI by itself) with different β-lactam antibiotics disc (penicillin, methicillin, cefoxitin, cefepime, and cefazolin). All three strains of MRSA were resistant to the tested antibiotics with no ZOI except for MRSA2, with an average ZOI of 8 mm for cefazolin (Figure 4). However, upon adding 4 μl of FGE to antibiotics, all the MRSA strains showed ZOI as shown in Figure 3. This data indicates that FGE has the ability to sensitize MRSA strains to β-lactam antibiotics to which they were resistant before.
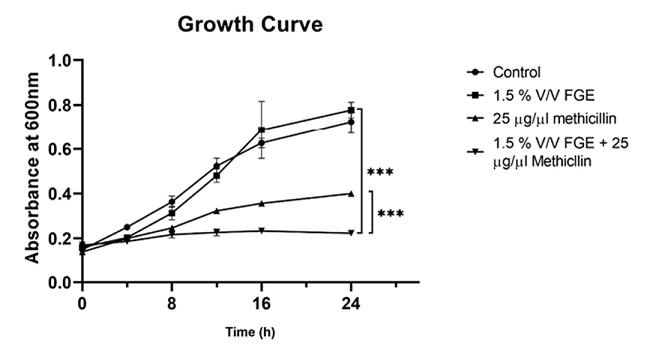
Figure 3. Growth curve analysis. Graph represents the growth of MRSA in the absence, presence of FGE and combination of FGE and methicillin as indicated in the graph. Standard deviations from triplicate experiments are indicated by errors bars and two-way ANOVA was performed to calculate the statistical significance between control and FEG + methicillin; and between methicillin and FEG + methicillin (*-P ≤ 0.05, **-P ≤ 0.01, ***-P ≤ 0.001, ****-P ≤ 0.0001).
Discussion
The study indicates that FGE inhibits biofilm formation by S. aureus, especially MRSA. This is a significant observation, as controlling MRSA biofilms could significantly reduce nosocomial MRSA infections. DAS, one of the organosulphur compound of garlic, could significantly reduce the MSSA and MRSA biofilm at a very low concentration. This suggests that organosulphur compounds are responsible for the inhibition of biofilm formation. The SEM images of MSSA and MRSA biofilm formed in the presence of FGE show a reduced number of bacteria suggesting that inhibition of biofilm formation by FGE could mostly be due to inhibiting attachment. It was intriguing to notice that FGE in combination with methicillin completely inhibited the growth of MRSA. In contrast, a very high concentration of methicillin could only partially retard the growth of MRSA. The ability of FGE to sensitize MRSA to methicillin underlines the potential of garlic and its compound to be used in combination with methicillin to treat MRSA infections. Similarly, FGE sensitized MRSA to different β-lactam antibiotics that it was initially resistant to. Research should focus on understanding the contribution of individual organosulphur compounds in FGE toward antibiofilm activity. Identifying garlic compounds and their mechanism of action of sensitization of MRSA to resistant antibiotics will enable their use in combination with antibiotics in clinical settings.