INTRODUCTION
In a generic way, when we talk about pneumonia, it is about an acute inflammatory process of the parenchyma pulmonary, caused by infectious agents, but also it may be caused by physical or chemical agents, well inhaled or by aspiration of gastric contents, when the level of consciousness is low or there is a swallowing disorder [1]. On the other hand, we define community-acquired pneumonia (CAP) as an inflammatory lesion of the pulmonary parenchyma that appears in response to arrival of microorganisms to the distal airway, which occurs in those immunocompetent people who have not been admitted to any institution. In clinical practice, assumes when there is “an acute infectious clinical presentation compatible and its radiological demonstration” [2,3].
CAP represents the origin of most sepsis and septic shock diagnosed in the Emergency Room (ER) [3] and is the main cause of death from infectious disease in developed countries and the first infectious cause (9%) of admission in the intensive care unit (ICU) [3]. It is attributed a global mortality of 10-14% according to age and associated risk factors; less than 1-2% in young people without comorbidity, 14% in hospitalized and about 25-50% in those admitted in the ICU [4,5].
The incidence of CAP ranges from 2-15 cases/1,000 inhabitants/year, being greater in smoking patients, in children under 5 years and in the elderly (>65 years) (up to 25-35 cases/1,000 inhabitants/year), with comorbidities, immunocompromised or enolic habit [4,5,6]. In the ER it diagnosis has increased from 0.85% of patients seen in 2001 to 1.35% in 2011 [6].
Approximately 75-80% of all CAPs are treated in the ER [6]. Of these, 40-60% will require hospital admission, including observation areas (with ranges very variable 22-65% according to centers, time of year and characteristics of patients), and of them between 2-10% will be in the ICU [6].
It is known the great variability among clinicians in the management of the diagnostic-therapeutic aspects in CAP [7,8], which it is one of the main reasons that explain the differences in admission rates from ER, the achievement of microbiological diagnosis, the request for complementary studies, the choice of antimicrobial pattern or diversity of applied care [7,8,9]. Therefore, it is the model of most relevant infection in the ER, so determine correctly the need for income, the location, and the intensity of care will condition the prognosis, mortality, request for tests and microbiological studies, the antibiotic pattern, the intensity of clinical observation and the use of socio-health resources (as well as its associated costs) [7]. In this sense, the implementation of clinical practice guidelines (CPGs) [8,9] prognostic severity scales (PSS) [7] and the new tools available in ERs such as biomarkers of inflammatory response and infection (BMIRI) [10,11], improve the adequacy of treatment [12].
ETIOLOGY
In general, the microbiological diagnosis is difficult to establish, being only identified the cause in 30-60% of the cases [1,2,4,9,13,14]. These scores are higher in severity CAPs due to the use of more diagnosis techniques. The isolations vary according to the severity of the CAP, the indication for outpatient treatment or hospital admission or in the ICU, and host factors both clinical and epidemiological [13,14].
Overall, the most frequent agent is Streptococcus pneumoniae (30-65%), being estimated that even in the 30-40% of cases not diagnosed by conventional methods the etiology is pneumococcal. Other common microorganisms are: Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, Haemophilus influenzae, influenza A virus, Coxiella burnetii, Chlamydophila psittaci, Staphylococcus aureus and bacilli gram negative [1,2,4,9,13,14].
It is known that the prognostic stratification of the CAP correlates with the etiology [14] (Table 1). This is usually monomicrobial except in aspiration pneumonia (multiple microorganisms of the oropharynx) [20,21]. Keep in mind that in 12-18% of CAP viruses appear involved, and in 8-14% are found pathogen associations ("mixed bacterial etiology": the majority S. pneumoniae plus M. pneumoniae or C. pneumoniae) [14]. The latter, together with the frequency of M. pneumonia (similar or greater than S. pneumoniae itself) in patients with home treatment, it will be necessary that any empirical oral treatment have adequate coverage and activity for both (S. pneumoniae and M. pneumoniae) [1,4,14].
Table 1. Etiology of community acquired pneumoniae.
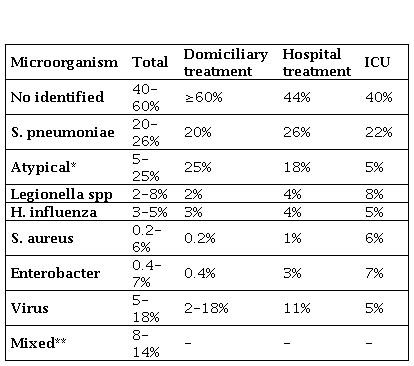
ICU: Intensive care unit. *Considering M. pneumoniae (the most frequent), C. pneumoniae, C. psittaci and C. burnetii. ** Most common associations: S. pneumoniae plus C. pneumoniae or M. pneumoniae.
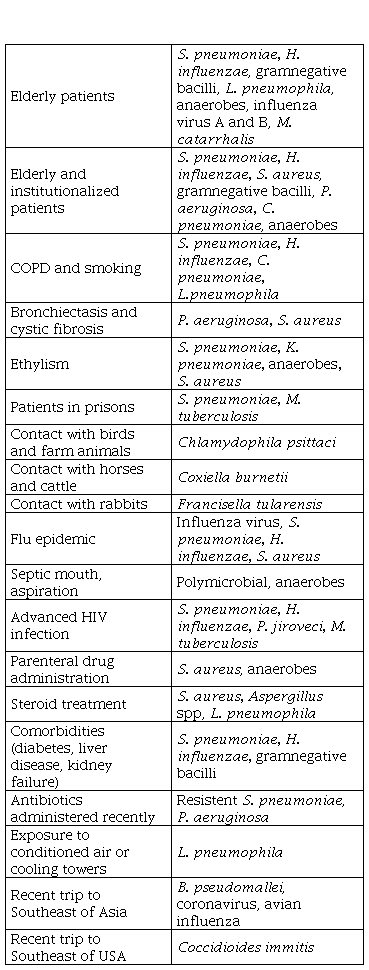
On the other hand, we must remember that there are a number of epidemiological conditions that predispose patients to suffer CAP due to certain pathogens [15] (Table 2).
ANAMNESIS, PHYSICAL EXPLORATION AND CLINICAL MANIFESTATIONS
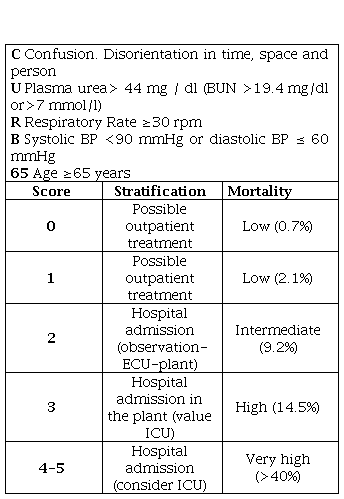
The general condition of the patient and his level of awareness must be evaluated, checking whether there are sepsis criteria [30,31]. We must look for signs of gravity, including dyspnea, tachypnea, cyanosis, use of accessory muscles, paradoxical breathing and edema [14]. Table 3 summarizes some of the criteria for hospital referral and probable admission.
Table 3. CAP: Community acquired pneumonia; SBP: Systolic blood pressure; mbp: Medium blood pressure: bpm: Beats per minute; CRB-65: acronym for confusion, respiratory rate ≥30, SBP <90 mmHg or diastolic (DBP) ≤60 mmHg and age ≥65 years.
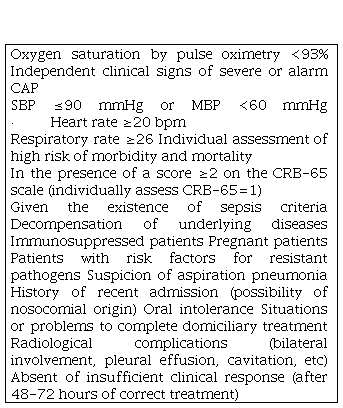
CAP: Community acquired pneumonia; SBP: Systolic blood pressure; mbp: Medium blood pressure: bpm: Beats per minute; CRB-65: acronym for confusion, respiratory rate ≥30, SBP <90 mmHg or diastolic (DBP) ≤60 mmHg and age ≥65 years.
The history will be performed whenever the clinical situation allows it. To reach a diagnosis of pneumonia is required first of all a detailed history that allows to put manifest related epidemiological or clinical conditions with specific pathogens (table 2) and thus classify the patient based on their prognostic factors, risk and associated underlying diseases [1,12]. In the interrogation will be done special emphasis on: age, baseline, recent antibiotic treatments, associated diseases, fever, cough, expectoration, pleuritic pain, suspicion of aspiration and comorbidity that needs treatment taking into account the drugs that take the patient at that time. The syndromic diagnosis of CAP is based on the existence of an acute infection clinic accompanied by a recent pulmonary infiltrate on the chest x-ray, not attributable to another cause.
In relation to the clinical manifestations, three syndromes are usually considered depending of the clinical-radiological presentation form (Table 4). Is differentiation between typical and atypical pneumonia not always is accepted by all authors nor is it clinically evident, especially in elderly and sick people with comorbidities, so which is becoming less decisive in the overall management of the process and its “utility is reduced to young adults without diseases associated ”[1,4,16].
Table 4. CAP syndromes depending on the clinical-radiological presentation form
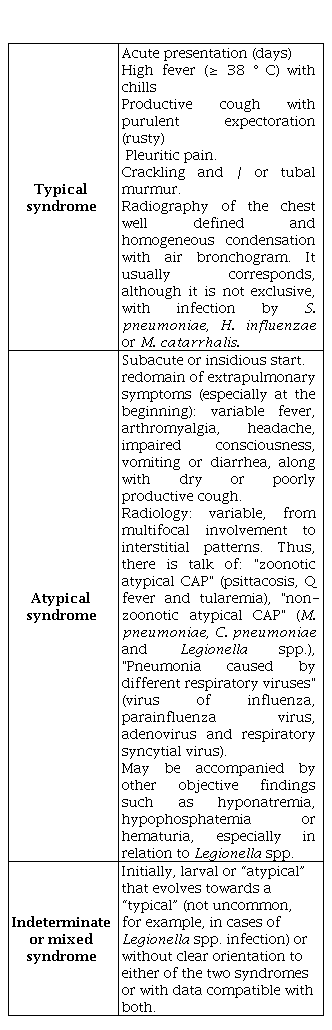
CAP: Community acquired pneumonia
In the case of the elderly, the form of presentation may be even more nonspecific and it is in them where we must increase the degree of suspicion: fever may be absent (due to the chronic use of anti-thermal or anti-inflammatory drugs), the expectoration is usually lacking and even the cough can be scarce [17,18]. It is not uncommon for the initial clinic of pneumonia in these patients is cognitive impairment, a fall, sphincter incontinence of recent onset or decompensation unexplained of their previous pathologies [17].
Although they are not specific to pneumococcal CAP, when two or more of the following criteria are presented, the chances of the causative bacteria being S. pneumoniae significantly increase: sudden onset fever and chills, pleuritic pain, purulent or rusty expectoration, cold sores, auscultation of tubal murmur, image of lobar condensation with aerial bronchogram on the thorax radiography, leukocytosis (>10,000 leukocytes/mm3) or leukopenia (<4,000 leukocytes/mm3) [1,2]. On the other hand, a concentration of Procalcitonin (PCT)> 0.85 ng/ml also forces to consider the pneumococcus as the etiological agent of the CAP [19,20].
COMPLEMENTARY STUDIES IN THE EMERGENCY ROOM
Complementary studies that should be performed on a patient with suspicion or confirmation of CAP depend largely on the estimated severity, and therefore on whether the management is going to be outpatient or hospital. They may also vary according to: the difficulty in guiding each case, the presence or absence of complications, the existence of individual circumstances and the clinical-epidemiological characteristics [2,4,15,16]. In the case of low-risk CAP with home treatment, antibiotic administration could be initiated without further evidence than radiography at the Health Center [21,22].
In order to unify the management of CAP in the ER, it is recommended, whenever there is availability, to request and evaluate [1,2,4,20]:
-
To all patients: posteroanterior and lateral chest radiography, hemogram and basic biochemistry [including glucose, ions, urea, creatinine, bilirubin, GOT (ATS), GPT (ALT)] and arterial blood gas [if Sat O2≤93% or the respiratory rate>20 breaths per minute (bpm) or there is cardiorespiratory comorbidity]. And if available, assess individually request in the ER: PCT and proadrenomedulin (proADM), as well as pneumococcal antigen and Legionella in urine.
To all those who enter and/or meet sepsis criteria (described in Table 3), in addition to the previous studies request: sputum culture, two blood cultures and urine antigens for pneumococcus and Legionella spp., coagulation study, lactate , PCT and proADM. And if influenza virus is suspected and/or treatment is indicated: nasopharyngeal aspirate.
If there is a significant pleural effusion, thoracentesis will be done requesting: pH, biochemistry, cells, Gram, culture. Assess pneumococcus and Legionella spp antigens and molecular biology techniques.
Individually and according to availability in certain circumstances (CAP that does not respond to the treatment or suspicion of resistant or infrequent pathogens) obtain samples for serologies (first sample) and other techniques such as Ziehl-Neelsen staining, mycobacterial culture, molecular techniques, culture for fungi, Giemsa or Kinyoun staining, etc.
Table 5 summarizes utilities and indications of the complementary studies.
Table 5. Complementary studies in the initial approach to pneumonia in the Emergency Department

PMN: Polymorphonuclear; CAP: Community acquired pneumonia.
In the clinical practice, the accessibility and speed to determine BMIRI in many ERs produce that these are postulated as added criteria to the PSS and to the clinical examination to assess the severity and improve decision-making when predicting the diagnosis of bacterial CAP [19,20], bacteraemia [23,24], as well as to decide the appropriate treatment [9]. In fact, the combination of PSS and BMIRI is considered today as the best strategy for prognostic assessment and prediction of mortality in patients with CAP [20]. Table 6 summarizes the recommendations and utilities for their use. Serum PCT is a more specific bacterial infection marker than C-reactive protein (PCR). PCT concentrations increase 4 hours after the onset of a bacterial infection and not in inflammation or viral infection. Thus, it may be useful for the diagnosis of bacterial infection in CAP [20]. But, it should be considered that a single value of PCT, especially in early stages, could be negative. PCT seriation offers greater diagnostic capacity [19]. Attending to proADM, it is a great predictor of mortality at 28-30 days, so it is being used in combination with prognostic scales and PCT to confirm bacterial involvement and decide patient admission [25].
Table 6. Recommendations on the use of biomarkers in patients affected of CAP in ER
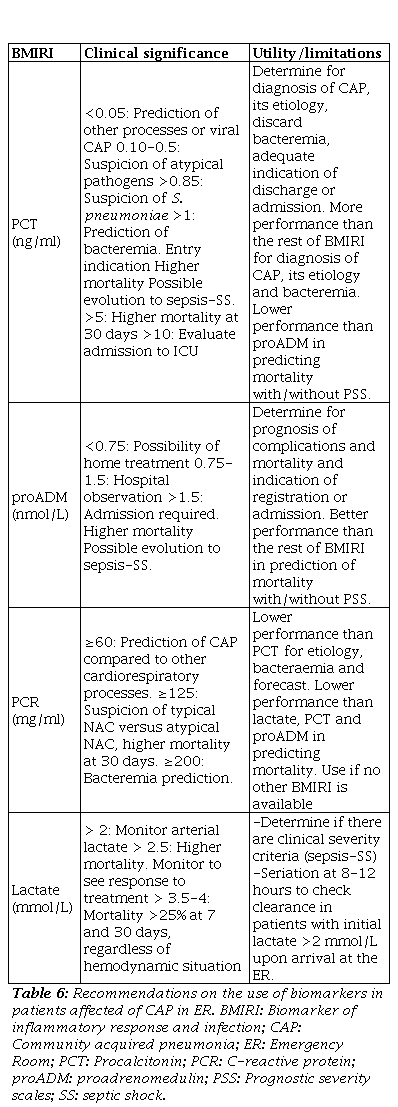
BMIRI: Biomarker of inflammatory response and infection; CAP: Community acquired pneumonia; ER: Emergency Room; PCR: Procalcitonin; PCR: C-reactive protein; proADM: Proadrenomedulin; PSS: Prognostic severity scales; SS: Septic shock.
Finally, attending to lactate as a marker of severity in the ER in CAP, its elevation indicates a state of tissue hypoperfusion (which may be associated with different etiologies, not only severe bacterial infection) that must be correlated with acidosis proven in gasometry [20,26].
PROGNOSTIC AND GRAVITY ASSESSMENT OF THE PATIENT WITH CAP
Although there are multiple PSS, the Fine or Pneumonia Severity Index (PSI) [27] and CURB-65 [28] scales are the most validated and recommended, and have been shown to have a similar ability to recognize patients at risk to die within 30 days [6]. Although, at present, the PSS known as SCAP (Severity Community Acquired Pneumonia) or "PS-CURX080" [29], is being used more frequently for the classification of risk it performs and for predicting severity, need for mechanical ventilation (MV) and possible evolution to septic shock and admission to the ICU.
PSI combines 20 items related with demographic, morbidity, exploratory, laboratory and radiological findings defining 5 risk classes (Table 7) in relation to 30-day mortality [27]. Depending on the assigned risk class, it recommends outpatient treatment (groups I-II), observation in the ER in class III, and hospital admission in classes IV-V. The PSI identifies well the low risk of mortality in classes I-III and helps us decide “discharge”, but may underestimate the severity, especially in young people with hypoxia, and does not assess additional criteria and circumstances that should be taken into account [2,7-9].
Table 7. PSI prognosis scale for pneumonia.
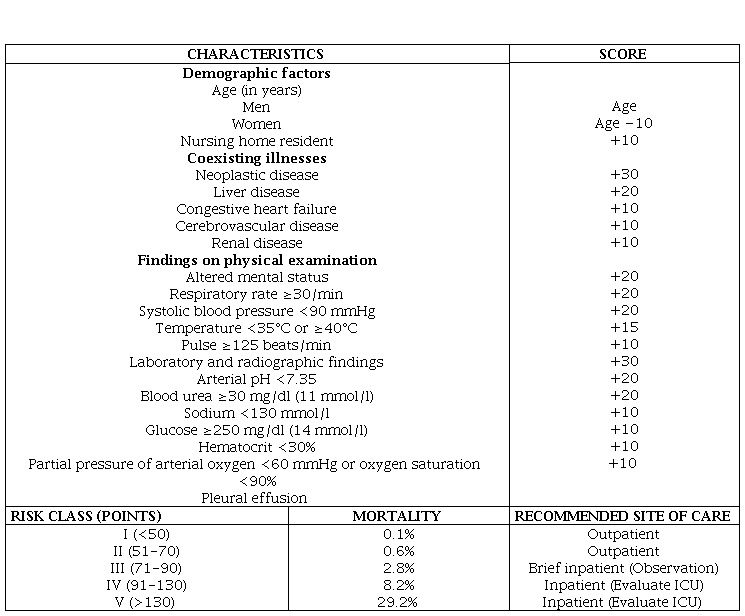
PSI: Pneumonia severity index; ICU: Intensive Care Unit.
Hence the concept of “Fine scale or modified PSI (PSIm)” [6], as a necessary update of the classic PSI, indicating the admission of low-risk patients (I-III) who present with respiratory failure (PaO2<60 mmHg) or concentrations of PCT>1 ng/ml (at least under observation) or any of the additional criteria indicated in Table 8. At his manner, most of the limitations and weaknesses of the PSI scale are saved. 16-27% of patients admitted to the ICU by CAP are initially classified with an I-III PSI and in them the most frequent reason for admission is due to respiratory failure (PaO2<60 mmHg and/or SatO2<90%). The use of the PSIm in the ER improves the adequacy of the income [6-9].
Therefore, in addition to the factors indicated in the PSS mentioned, which confer a punctual and static assessment of the CAP, and those dependent on the patient's own functional status, other independent and dynamic additional criteria must be taken into account, such as the infection itself and the systemic inflammatory response, which influence and determine the prognosis in the first hours of the patient's stay in the ER [5,7,19]. Among these are: the estimation of the probability of bacteraemia, the existence of sepsis or septic shock as stages of a dynamic process, and the inclusion of BMIRI to collaborate in the decision of admission and/or more appropriate location [6,20].
The clinical situation of the patient with CAP, according to the criteria of sepsis and septic shock is essential [30,31], and determines that the patient should be reassessed after a few hours (8-12-24), and, by therefore, at least, remain under observation of the ER [6]. In addition, the frequency of bacteraemia increases with the severity of the clinical picture (17-31% in sepsis and 30-45% in septic shock) [32]. Although all vital signs have been associated as individual predictors of mortality [(RR≥26 rpm, HR≥120 bpm, Tª>38.3 °C and SBP≤90 mmHg]. SBP is the best marker, as a hemodynamic sign, independent predictor 30-day mortality and the need for MV and/or support with inotropic agents [29,33]. It is important to remember that a constant search for clinical evidence prior to hypotension is necessary to identify the serious patient, since although SBP is a good prognostic marker, it is not an early shock [34]. In fact, the new sepsis criteria (SOFA) are not sufficient in patients who classify with low risk to predict a bad evolution [35], that if they can advance BMIRI [20].
Due to that, the British Thoracic Society (BTS) prepared the CURB-65 scale [28], acronym for confusion, urea>44 mg/dl, RR≥30 rpm, SBP<90 mmHg or DBP≤60 mmHg and age≥65 years, defining 6 risk groups (Table 9). It better detects high-risk patients (classes 3-5) who should be admitted, but it also has great limitations, among which are the power to overestimate and indicate admission in many of those over 65 by the criteria of age, which should not be the only indicator of income at present, nor does it value oxygen saturation or PaO2 [6]. The assessment of "confusion" may be done with a questionnaire of ten questions or simply assessing the appearance of disorientation in time, space or person. The calculation is made by adding 1 point for each variable present with a range between 0-5 points [28].
In Primary Care, the CRB-65 scale [4,7,22], acronym for confusion, RR≥30 rpm, SBP<90 mmHg or DBP≤60 mmHg and age≥65 years is recommended, so that admission would be indicated (therefore hospital referral as previously indicated) with≥2 criteria. In cases with CRB-65=1, it should be assessed individually (and take into account the existence of criteria or situations included in tables 3 and 8) [7,21,22].
In addition, an individual assessment must be made in each case by the emergency physician, and that is why most of the guidelines recommend following 3 steps to decide the admission or home treatment of the CAP [4,7]:
Assess possible conditions that hinder or compromise home care (social or psychiatric problems that make suspect poor treatment compliance or intolerance to oral treatment or respiratory failure) and the so-called additional criteria.
-
Once the foregoing has been assessed, evaluate the risk in the PSIm or CURB-65 prognostic scales.
Finally, a judicious clinical evaluation should be applied with all the available elements including the characteristics and possibilities of each hospital (existence or not of observation, consultations, day hospital, etc.), opting in doubtful cases for the safer decision for the patient. Cases of patients with CAP that meet sepsis criteria should at least remain under observation to see their immediate evolution [7,30].
Other PSS have emerged in recent years. Among them, the one known as SCAP (Severity Community Acquired Pneumonia) or “PS-CURXO80” [29], which contains 2 major and 6 minor variables and is already used in multiple centers and recommended by many experts. This is because, in addition to predicting mortality as does the PSI and CURB-65, it has been validated and is able to predict the need for MV and evolution to septic shock [7]. The SCAP scale defines a CAP as severe (SCAP) if the patient has at least one major criterion or two minor criteria [29].
TREATMENT
The difficulty in the etiological diagnosis means that in several cases an empirical treatment is indicated, except when the microbiological diagnosis is confirmed in the ER, which allows us to establish a targeted treatment. The therapeutic recommendations are generally established according to the PSI classification and the fate of the patient decided [2,36-38].
Regardless of the pattern and the indicated antimicrobials, the first appropriate doses of antibiotic should always be administered as early as possible in the ER itself (immediately if there is sepsis or septic shock), which decreases hospital stay and mortality in both patients mild as in those who present with sepsis or with septic shock [39].
Table 10 shows the recommendations of empirical treatment orally (po) or intravenously (iv) according to the patient's destination. This table will be applicable for the majority of CAP cases treated in ER [1,4,15,36-38]. On the other hand, Table 11 summarizes the treatment recommendations in special situations [4,21,36-41]. If the antigenic study of seasonal flu is positive, oseltamivir 75 mg/12 hours will be indicated [36].
Table 10. Recommendations for empirical antimicrobial treatment in CAP according to PSI score.

CAP: Community acquired pneumonia; PSI: Pnemonia Severity Index; por: Orally; iv: Intravenous; h: Hours.
In addition, some important considerations in the election of the antimicrobial pattern in the CAP:
The decision of the antibiotic regimen (monotherapy or combination therapy) must take into account the antimicrobials administered in the three months prior to the patient to select a different class of antimicrobials, as well as the severity of the clinical situation that the combination therapy could recommend up to the isolation of the etiologic agent or the improvement of the patient [1,4].
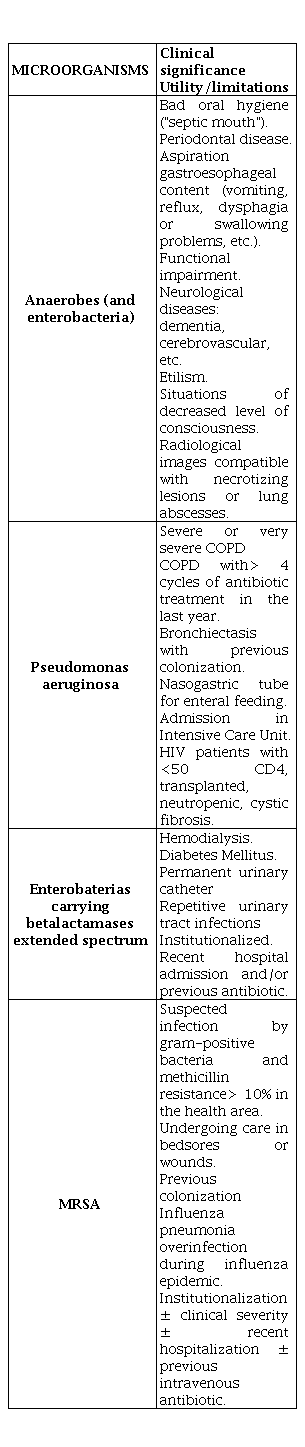
In order to establish antimicrobial treatment, the existence of risk factors for resistant pathogens should be considered (Table 12) that may change the antibiotic pattern decision [4,41-44].
In relation to the "concentration-dependent antibiotics": within this group, looking for the most appropriate option, we must point out that moxifloxacin (400 mg/24 h) is 4-8 times more active than levofloxacin against S. pneumoniae. Although the serum concentration of levofloxacin (Cmax) is higher than that of moxifloxacin, to obtain a value of the area under the curve similar to that of this one, levofloxacin should be administered at doses of 500 mg/12 h [1,45]. The exposure time during the 24 h of the day or area under the achieved inhibitory curve (ABC24/MIC), is transcendental to estimate the clinical efficacy, since, the greater this is (for S. pneumoniae it should always be≥30 mg/h/l), will increase clinical success and decrease the possibility of development of mutants and resistance, a crucial fact that occurs with moxifloxacin orally (according to the CMIs its ABC24/MIC is between 96-384 mg/h/l) , while for levofloxacin or azithromycin (orally) they are 35 and 3 mg/h/l, respectively [14,60-62].
In relation to "time-dependent antibiotics": for aminopenicillins and cephalosporins it is necessary that at least the T>MIC (time on the MIC) is 40-50% of the time between two doses of the drug to be effective. Within this group and against S. pneumoniae, cefditoren is several times more active than amoxicillin-clavulanic, although in practice the PK-PD (pharmacokinetic/pharmacodynamic) parameters of both are superimposable with doses of 400 mg/12 h of cefditoren and 2,000/125 mg/12 h dose of the delayed amoxicillin-clavulanic formulation for 10 days for a CAP. Thus, according to its MICs, the foreseeable in vitro activity of cefditoren is 94% with doses of 200 mg/12 h and 99.8% at doses of 400 mg/12 h, which makes this last guideline the best option among cephalosporins orally [45-47].
In elderly patients avoid the use of fluoroquinolones if there is a risk of infection by enterobacter due to the high percentage of resistance [36].
Table 11. Recommendations for antimicrobial treatment in CAP in special situations.
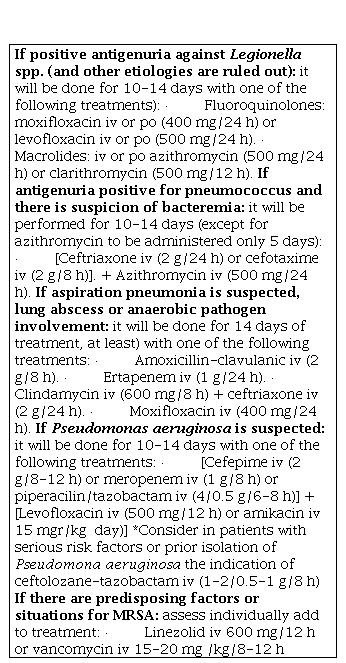
CAP: Community acquired pneumonia; PSI: Pneumonia Severity Index; po: orally; iv: intravenous; h: hours; MRSA: Methicilin resistant S. aureus