INTRODUCTION
Paramedian forehead flap was first described by Indian physician Sushruta in 500 bc to reconstruct nasal defects from punitive tip amputation as punishment for theft and adultery. Orbital exenteration is a disfiguring procedure that leaves a large defect and significant morbidity to the patient. Although free flaps are the best option to be used to cover the defect, it is a challenge to a patient who had previous radiotherapy treatment to the head and neck region due to its late vascular complication. We present a case of post-orbital exenteration who is not suitable for a free flap and wound coverage was done with a paramedian forehead flap as an alternative.
CASE REPORT
A 63-year-old female who is a known hypertensive on treatment was diagnosed with nasopharyngeal carcinoma in 1996. She had completed radiotherapy and was disease-free since then. However, she presented twelve years later with bulging of the right eye which was worsening over the course of a few months. It was associated with blurring vision over the right eye, however, there were no signs and symptoms of increased intracranial pressure such as severe headache, fitting or persistent vomiting. On examination, she was found to have right eye proptosis and her visual acuity was that she could only perceive light.
A computed tomography (CT) guided biopsy of the right orbital mass was taken and histopathological examination showed lymphoepithelial carcinoma. Contrast-Enhanced Computer Tomography (CECT) of orbit revealed well defined soft tissue mass in the right orbit, and the inferior rectus muscle was elevated by a mass. The mass compressed the posterior part of the inferior, medial and lateral rectus muscles and there was no clear margin seen. The posterior part of the optic nerve was also compressed by the mass. The mass has extended into the optic canal with a widening of the canal measuring 0.42cm compared to the contralateral side (0.2cm). However, the superior rectus muscle was normal. The superior ophthalmic vein was mildly dilated and cavernous sinuses were normal. There were no distant metastases.
Multidisciplinary team discussion was carried out among the neurosurgeon, ophthalmologist and otorhinolaryngology surgeon. Based on the clinical features and CECT findings, a consensus was made and she underwent right eye exenteration, right craniotomy plus excision of the tumor, and middle maxillary antrostomy. Subsequently, the histopathological examination came back as consistent with metastatic nasopharyngeal carcinoma with a clear margin. Immunohistochemistry was positive for CK; however, S100 and HMB45 were negative.
Unfortunately, postoperatively, she had a reduced level of consciousness and an emergency CT brain revealed increasing pneumocranium and cerebral edema. An emergency re-craniotomy, dura repair with bilateral subdural drain, and external lumbar drain insertion were performed. The oculoplastic team attempted for coverage of the orbital defect with a split-thickness skin graft in the same setting, however, the procedure failed. At this point, the patient was referred to the plastic and reconstructive surgery team for a vascularized soft tissue transfer for an intraorbital barrier.
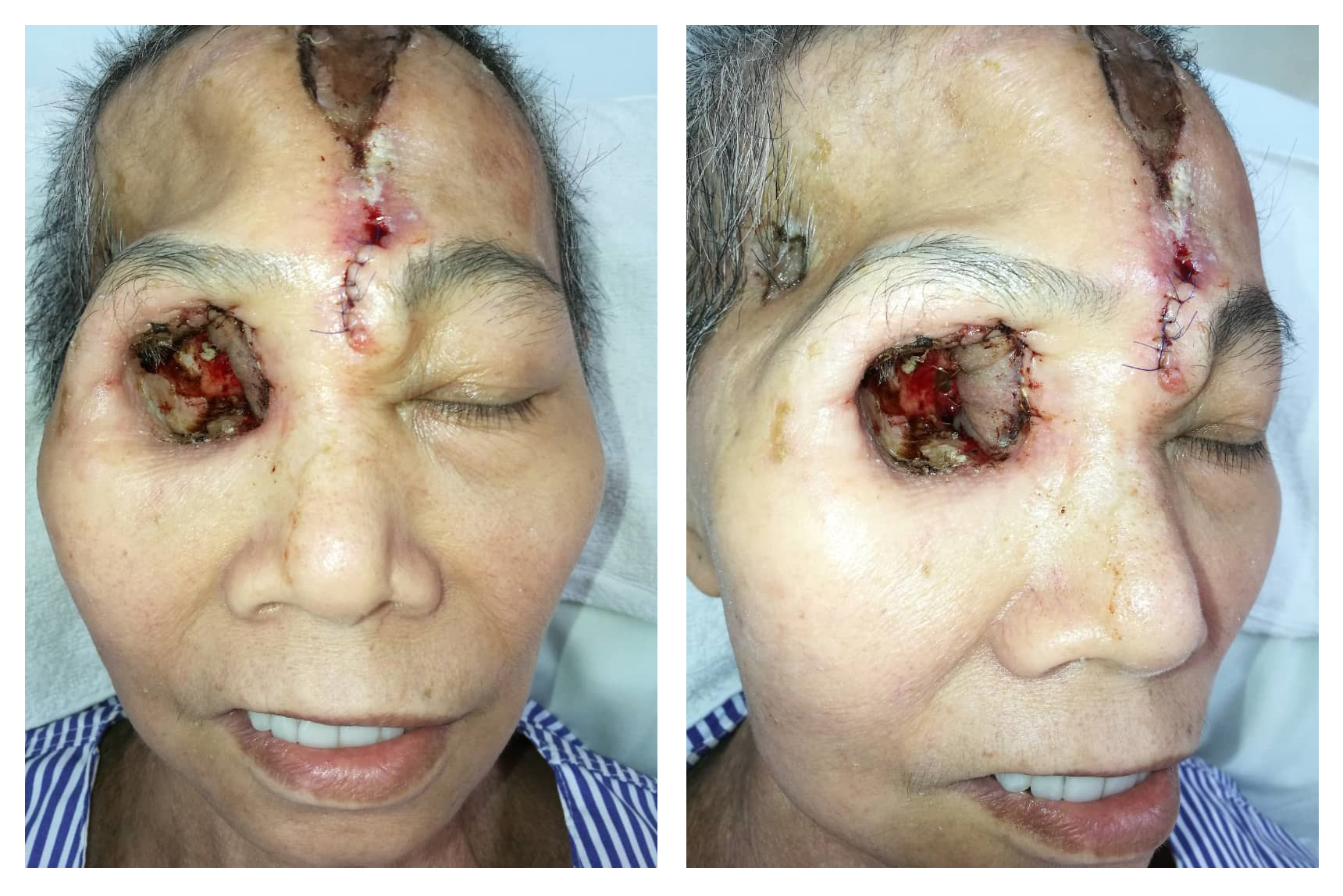
The patient was counseled for free radial forearm flap to cover the orbital wall defect and she agreed. During the surgery, a right orbital defect post exenteration of right orbit with communicating wound into the right cranium and right maxillary antrum was observed. Right optic canal, nasolacrimal canal, superior and inferior orbital fissure was also exposed (Figure 1). We explored the right facial artery and vein to be used as the recipient's vessel for anastomosis. However, both vessels were caked from the previous radiotherapy effect. The facial artery was non-pulsatile and after transection, there was very poor backflow despite a systolic BP of 160 mmHg. A decision was made to abandon the free flap procedure and we opted for contralateral paramedian forehead flap as an alternative method of coverage due to poor doppler signal of the ipsilateral supraorbital and supratrochlear arteries. After the left paramedian forehead flap was raised, it was rotated anti-clockwise and inserted into the right orbital cavity to cover most of the defects (Figure 2). The flap was anchored using Vicryl 4/0 and flap division was done in stages as a method of flap delay. The donor site was covered with a split-thickness skin graft harvested from the thigh. A complete flap division was performed 3 weeks later. The final outcome of the surgery is that we managed to cover the optic foramen and superior orbital fissure, therefore, reducing the risk for ascending infection into the brain (Figure 3).
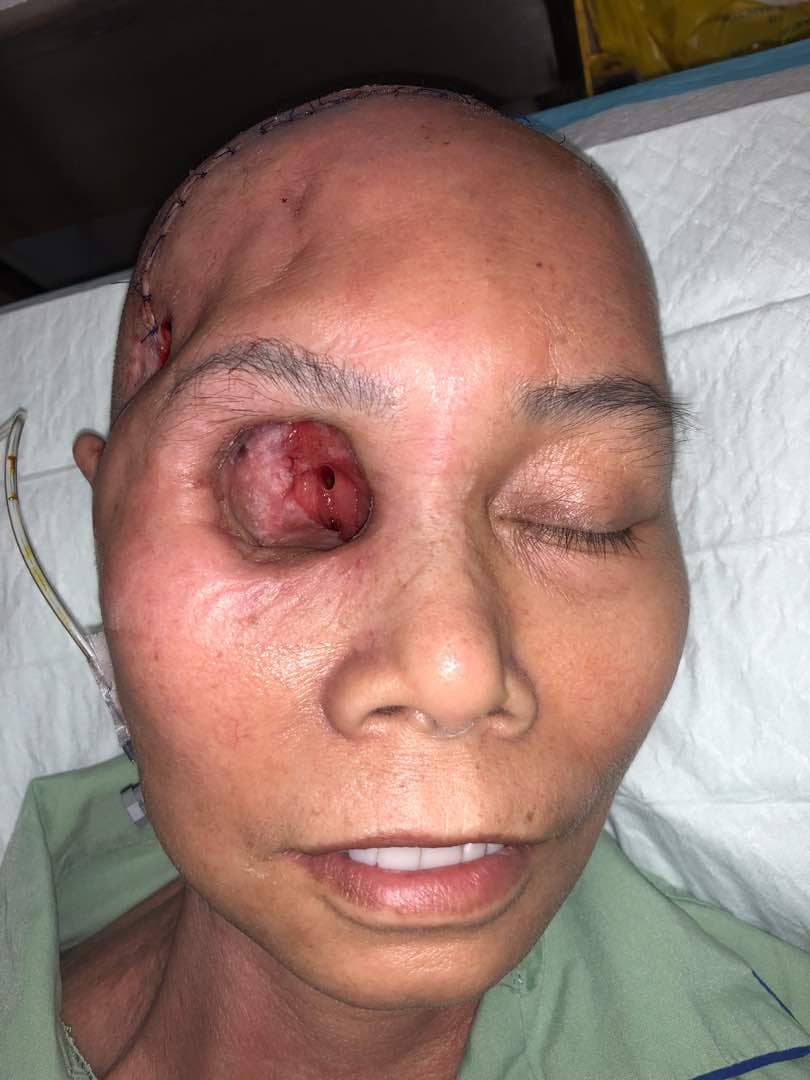
Figure 1. Intraorbital wall defect post exenteration of the right orbit exposing the right optic canal surrounded by granulation tissues.
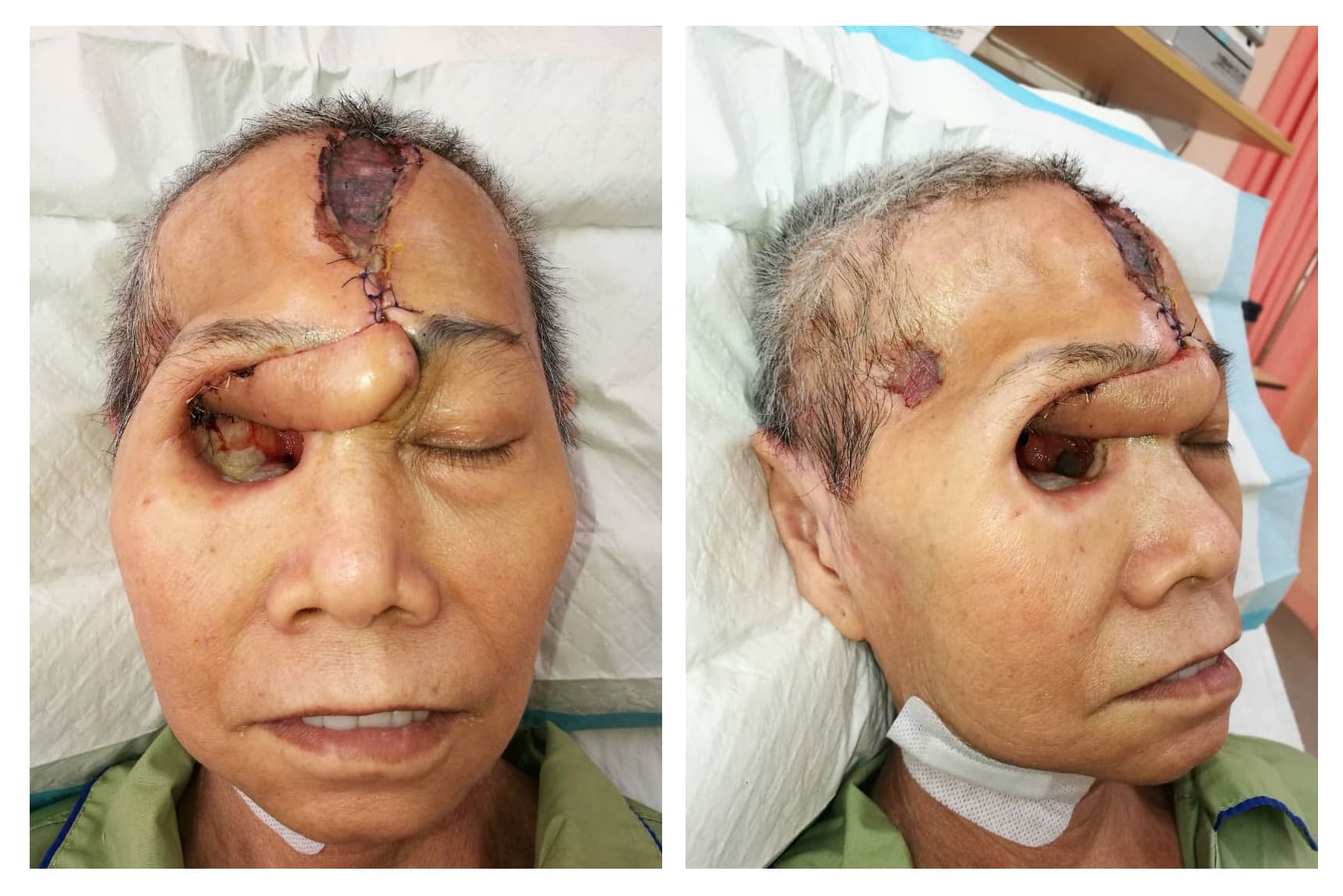
Figure 2. 1-week post paramedian forehead flap. The superior part of the donor site is covered with a split-thickness skin graft and the inferior part is closed primarily.
DISCUSSION
Reconstructing orbital cavity post-orbital exenteration can be quite challenging even to an experienced surgeon. The main goal of the reconstruction is to obliterate any connection between the orbit and the intracranial compartment while providing a pleasant cosmetic outcome. The donor tissue that is going to be used for reconstructive purposes should resemble the color and texture of the site of coverage while reducing donor site morbidity.
One of the best options for orbital socket coverage is via a free tissue transfer. This is due to the fact that there is no limit to the volume of the tissue that can be transferred effectively to the orbit provided that the recipient site has a good recipient vessel for anastomoses. However, patients with orbital wall defect post tumor excision often undergo adjuvant chemotherapy or irradiation which could compromise the integrity of the vessels surrounding the area.
Several studies have discussed the outcome of the microvascular anastomosis in a post-irradiated area which suggests that the patency rates of the irradiated vessels are low [1-2]. Despite that, most clinical studies show there is no difference in the viability of the flap between non-irradiated and irradiated cases [3-4]. Therefore we initially planned to reconstruct the orbital wall defect using a free fasciocutaneous radial forearm flap based on the above evidence.
Preoperatively, flap mapping was done over the right forearm as the donor tissue and the course of the right facial artery identified using a handheld doppler. Both arterial and venous signals were good at the recipient site and the patient was then prepped for surgery. Intraoperatively, the right facial artery was identified, skeletonized and divided. However, the artery’s wall was found to be thickened, lumen narrowed and the backflow was poor. This could be due to late radiotherapy complication that induces vascular inflammatory changes that led to the prothrombotic state of the endothelium [5]. Some authors have also theorized that prolonged interval (> 15 weeks) between the radiotherapy and the timing of surgery will adversely affect the outcome of the microvascular complication in free flap reconstruction of the head and neck region [6].
Since the recipient's vessel is not suitable for microvascular anastomosis due to the above factor, we decided to convert to a local flap coverage using a fasciocutaneous paramedian forehead flap. Forehead flap was invented by an Indian Physician Sushruta approximately in 500 BC. Back then, it was popularly used to reconstruct a nasal defect post nose amputation [7]. In the 1830s, Warren first performed a forehead flap in America. But it was Kazanjian who further refined forehead flap by describing for the first time the primary blood supply of the forehead via supratrochlear and supraorbital arteries [8]. The advantage of forehead flap is that it has an axial pattern blood supply which leads to predictable flap survival and thus obviates the need for microvascular anastomosis. The forehead skin also closely matches the color and texture of the periorbital tissue and most importantly, it is pliable and can be trimmed to conform to the complex landscape around the orbit [9].
CONCLUSIONS
In a scenario where a free tissue transfer is not an option for the reconstruction of the periorbital defects due to various factors, forehead flaps are proven to be a reliable safe boat and highly effective choice. This is due to the robust blood supply which increases the success rate and the relative ease to harvest the flap leading to short operative time. They also have the added advantage of providing similar skin texture and color match to those at the recipient bed and significantly improve the cosmetic outcome postoperatively.