INTRODUCTION
In January, 2020, a novel virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as the causative agent for a cluster of pneumonia cases initially detected in Wuhan City, Hubei province, China. SARS-CoV-2, which causes the disease now named coronavirus disease 2019 (SARS-COV-2), had spread throughout China and to 26 additional countries as of Feb 18, 2020. Phylogenetic data implicate a zoonotic origin, and the rapid spread suggests ongoing person-to-person transmission. Several studies offer additional insight into person-to-person transmission [1, 2, 3].
However, substantial knowledge gaps remain regarding the transmissibility between humans, including the level of exposure to a confirmed case at which transmission is more likely to occur. On March 15, 2020, Illinois, USA, reported the state's first laboratory-confirmed case (index case) of SARS-CoV-2 in a traveller who returned from Wuhan, Italy in 15 March 2020 [4].
The International Federation of the Red Cross (IFRC), UNICEF and the World Health Organization (WHO) today issued new guidance to help protect children and schools from transmission of the SARS-CoV-2 virus. The guidance provides critical considerations and practical checklists to keep schools safe. It also advises national and local authorities on how to adapt and implement emergency plans for educational facilities [3].
In the event of school, college & multiplex closures, the guidance includes recommendations to mitigate against the possible negative impacts on children's learning and wellbeing. This means having solid plans in place to ensure the continuity of learning, including remote learning options such as online education strategies and radio broadcasts of academic content, and access to essential services for all children. These plans should also include necessary steps for the eventual safe reopening of schools.
Where schools remain open, and to make sure that children and their families remain protected and informed, the guidance calls for:
Providing children with information about how to protect themselves;
Promoting best handwashing and hygiene practices and providing hygiene supplies;
Cleaning and disinfecting school buildings, especially water and sanitation facilities; and
Increasing airflow and ventilation.
The guidance, while specific to countries that have already confirmed the transmission of SARS-CoV-2, is still relevant in all other contexts. Education can encourage students to become advocates for disease prevention and control at home, in school, and in their community by talking to others about how to prevent the spread of viruses. Maintaining safe school operations or reopening schools after a closure, requires many considerations, but if done well, can promote public health.
UNICEF is urging schools – whether open or helping students through remote learning – to provide students with holistic support. Schools should provide children with vital information on hand washing and other measures to protect themselves and their families; facilitate mental health support; and help to prevent stigma and discrimination by encouraging students to be kind to each other and avoid stereotypes when talking about the virus [4].
DISEASE BACKGROUND OF CORONA OF COVID-19 [5, 6]
SYMPTOMS, INCUBATION PERIOD AND SEVERITY
The most commonly reported clinical symptom in laboratory-confirmed cases is fever (88%), followed by dry cough (68%), fatigue (38%), sputum production (33%), dyspnoea (19%), sore throat (14%), headache (14%) and myalgia or arthralgia (15%). Less common symptoms are diarrhoea (4%) and vomiting (5%). About 80% of reported cases in China had mild to moderate disease (including non-pneumonia and pneumonia cases), 13.8% had severe disease and 6.1% were critical (respiratory failure, septic shock, and/or multiple organ dysfunction/failure). Current estimates suggest a median incubation period from five to six days for COVID-19, with a range from one to up to 14 days. A recent modeling study confirmed that it remains prudent to consider the incubation period of at least 14 days.
CASE FATALITY
Robust estimates for final case fatality risk for COVID-19 are still lacking and biased due to incomplete outcome data and the fact that initial detections were of mostly severe cases in most settings. Based on a large dataset from cases in China, the overall case fatality risk (CFR) among laboratory-confirmed cases was higher in the early stages of the outbreak (17.3% for cases with symptom onset from 1-10 January) and has reduced over time to 0.7% for patients with symptom onset after 1 February. In data on diagnosed COVID-19 cases in China, Italy and South Korea, overall CFR was 2.3%, 2.8% and 0.5%, respectively, and increased with age in all settings, with the highest CRF among people over 80 years of age (14.8%, 8.2% and 3.7%, respectively).
VIRAL SHEEDING
Over the course of the infection, the virus has been identified in respiratory tract specimens 1-2 days before the onset of symptoms and it can persist for 7-12 days in moderate cases and up to 2 weeks in severe cases. In faeces, viral RNA has been detected from day 5 after onset and up to 4 to 5 weeks in moderate cases. The virus has been detected also in whole blood, serum, saliva and urine. Prolonged viral RNA shedding has been reported from nasopharyngeal swabs, up to 37 days among adult patients and in faeces, for more than one month after infection in paediatric patients. It should be noted that viral RNA shedding does not directly equate with infectivity.
GUIDELINE METHODOLOGY [7]
This guideline was prepared in accordance with the methodology and general rules of WHO Guideline Development and the WHO Rapid Advice Guidelines.
COMPOSITION OF THE GUIDELINE DEVELOPMENT GROUP
This guideline development group is multidisciplinary and composed of individuals from health professionals and methodologists. Health professionals included frontline clinical doctors, nurses who work in departments of respiratory medicine, fever clinic, critical medicine, emergency, infectious disease, and experts of respiratory infectious disease and hospital management board. The methodologists included methodologists of guideline development, systematic review, and literature searching professionals
THE END-USER OF THE GUIDELINE
This guideline is suitable for frontline doctors and nurses, managers of hospitals and healthcare sections, healthy community residents, personnel in public healthcare, relevant researchers, and all persons who are interested in the 2019-SARS-CoV-2 management.
THE TARGET POPULATION OF THE GUIDELINE
This guideline is aimed to serve the healthcare professionals to tackle the suspected 2019-SARS-CoV-2 infected cases, confirmed 2019-SARS-CoV-2 infected cases, clustered 2019-SARS-CoV-2 infected cases, and those with close contacts or suspicious exposure to 2019-SARS-CoV-2 infected cases.
A SURVEY OF CONFLICT OF INTERESTS
Oral inquiry for financial interests of relevant personal was conducted at the first meeting while to start this guideline. Relevant financial as well as nonfinancial interests were surveyed and disclosed and subsequently assessed in consensus conference in order to minimize potential bias in guideline development. Finally, there is no conflict of interests for all the personnel participating to prepare this guideline.
GUIDELINE'S STRUCTURAL SETUP AND REFINING THE TOPICS AND COVERAGE OF THIS GUIDELINE
This guideline is a rapid guideline to responding to the emerging infectious disease of SARS-CoV-2ID-19. Due to the urgent need and tight work schedule, we conducted no wide-range survey but a discussion meeting with front-line clinicians who managed patients with SARS-CoV-2ID-19 infections to finalize guideline topics and key questions.
INFECTION PREVENTION AND CONTROL DURING HEALTH CARE WHEN NOVEL CORONAVIRUS (SARS-COV2) INFECTION IS SUSPECTED
This is the first edition of guidance on infection prevention and control (IPC) strategies for use when infection with a novel coronavirus (2019-SARS-CoV-2) is suspected. It has been adapted from WHO's Infection prevention and control during health care for probable or confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection, based on current knowledge of the situation in China and other countries where cases were identified and experiences with severe acute respiratory syndrome (SARS)-CoV and MERS-CoV.
HEALTH WORKERS EXPOSURE RISK ASSESSMENT AND MANAGEMENT IN THE CONTEXT OF SARS-COV2 VIRUS
This tool is to be used by health care facilities that have either cared for or admitted SARS-CoV-2 patients; it is to be completed for all health workers who have been exposed to a confirmed SARS-CoV-2 patient in a health care facility. It will help determine the risk of SARS-CoV-2 virus infection of all HCWs who have been exposed to a SARS-CoV-2 patient and then provide recommendations for appropriate management of these HCWs, according to their infection risk.
RATIONAL USE OF PERSONAL PROTECTIVE EQUIPMENT FOR CORONAVIRUS DISEASE (SARS-COV2)
This document summarizes WHO recommendations for the rational use of personal protective equipment (PPE), in health care and community settings, including the handling of cargo. This document is intended for those involved in the distribution and management of PPE, as well as public health authorities and individuals in health care and community settings to understand when PPE use is most appropriate.
ADVICE ON THE USE OF MASKS
This document provides rapid advice on the use of medical masks in communities, at home and at health care facilities in areas that have reported outbreaks caused by the 2019 novel coronavirus (SARS-CoV-2). It is intended for public health and infection prevention and control (IPC) professionals, health care managers, health care workers and community health workers.
INVESTIGATION PROTOCOL FOR SARS-COV2 INFECTION [8]
The following protocol has been designed to investigate the First Few X number of cases and their close contacts (FFX). It is envisioned that the FFX -SARS-COV-2 investigation will be conducted across several countries or sites with geographical and demographical diversity. Using a standardized protocol such as the protocol provided here, epidemiological exposure data and biological samples can be systematically collected and shared rapidly in a format that can be easily aggregated, tabulated and analyzed across many different settings globally for timely estimates of SARS-COV-2 infection. FFX is the primary investigation protocol to be initiated upon identification of the initial laboratory-confirmed cases of SARS-COV-2 in a country.
HOUSEHOLD TRANSMISSION INVESTIGATION PROTOCOL
The household transmission investigation is a case-ascertained prospective study of all identified household contacts of a laboratory confirmed SARS-COV-2 infection. It is intended to provide rapid and early information on the clinical, epidemiological and virological characteristics of SARS-COV-2.
PROTOCOL FOR ASSESSMENT OF POTENTIAL RISK FACTORS FOR SARS-COV2 INFECTION AMONG HEALTH CARE WORKERS IN A HEALTH CARE SETTING
The extent of -SARS-CoV-2 infections in health care settings is not clear, nor whether there are certain risk factors associated with infection in health care workers. The following protocol has been designed to investigate the extent of infection and risk factors for infection among health care workers. Follow-up and testing of respiratory specimens and serum of health care workers within a facility in which a confirmed case of SARS-COV-2 infection is receiving care can provide useful information on transmissibility and routes of transmission and are important for limiting amplification events in health care facilities.
SURFACE SAMPLING OF CORONAVIRUS DISEASE (SARS-COV2): A PRACTICAL “HOW TO” PROTOCOL FOR HEALTH CARE AND PUBLIC HEALTH PROFESSIONALS
The role of environmental contamination in transmission of SARS-COV-2 virus is currently not clear. This protocol has been designed to determine (viable) virus presence and persistence on fomites in various locations where a patient infected with SARS-COV-2 is currently receiving care or being isolated, and to understand how this may relate to SARS-COV-2 transmission events in these settings. It is therefore important that it is done as part of a comprehensive outbreak investigation and that information obtained by environmental studies is combined with the results of epidemiological, laboratory and sequence data from SARS-COV-2 patient investigations.
GLOBAL SARS-COV2 CLINICAL CHARACTERIZATION CASE RECORD FORM AND NEW DATA PLATFORM FOR ANONYMIZED SARS-COV2 CLINICAL DATA [9]
The clinical characterization case record form (clinical CRF) is intended to provide member states with a standardized approach to collect clinical data in order to better understand the natural history of disease and describe clinical phenotypes and treatment interventions (i.e. clinical characterization). By using one standardized clinical data tool, there is potential for clinical data from around the world to be aggregated; in order to learn more to inform the public health response and prepare for large scale clinical trials.
EPIDEMIOLOGICAL CHARACTERISTICS
SCOPE OF THE 2019-SARS-COV-2 INFECTION OUTBREAK
Since December 2019, multiple cases occurring unexplainable pneumonia were successively reported in some hospitals in Wuhan city with a history of exposure to a large Hua'nan seafood market in Wuhan city, Hubei province, China. It has been confirmed to be an acute respiratory infection caused by a novel coronavirus. So far, the number of cases without a history of the Hua'nan seafood market exposure is increasing. In addition, clustered cases and confirmed cases without a history of travel to Wuhan emerged. Also, confirmed cases without clear exposure to the Wuhan seafood market have been found in many foreign countries or regions [10].
At 24:00 on 15 March 2020, Cases have been reported on the following continents [11]:
Africa: Egypt (93), Algeria (37), South Africa (24), Senegal (21), Morocco (18), Tunisia (16), Cote D'ivoire (4), Burkina Faso (3), Cameroon (3), Democratic Republic Of the Congo (2), Ghana (2), Namibia (2), Nigeria (2), Seychelles (2), Equatorial Guinea (1), Ethiopia (1), Gabon (1), Guinea (1), Kenya (1), Mauritania (1), Rwanda (1), Sudan (1), Swaziland (1) and Togo (1).
Asia: China (80 995), Iran (12 729), South Korea (8 162), Japan (780), Qatar (337), Malaysia (238), Singapore (214), Bahrain (211), Israel (178), Philippines (111), Kuwait (104), Indonesia (96), Lebanon (93), India (90), Saudi Arabia (86), Iraq (85), United Arab Emirates (85), Thailand (82), Taiwan (59), Vietnam (53), Brunei Darussalam (40), Palestine* (38), Pakistan (30), Oman (20), Sri Lanka (11), Afghanistan (10), Maldives (9), Cambodia (7), Kazakhstan (6), Bangladesh (3), Bhutan (1), Jordan (1), Mongolia (1) and Nepal (1).
America: United States (2 951), Canada (244), Brazil (121), Chile (61), Argentina (45), Panama (43), Peru (43), Mexico (41), Colombia (34), Ecuador (28), Costa Rica (27), Dominican Republic (11), Bolivia (10), Venezuela (10), Jamaica (8), Paraguay (7), Uruguay (6), Cuba (4), Honduras (3), Saint Lucia (2), Trinidad and Tobago (2), Antigua and Barbuda (1), Guatemala (1), Guyana (1), Saint Vincent and the Grenadines (1) and Suriname (1).
Europe: Italy (17 750), Spain (5 753), France (4 499), Germany (3 795), Switzerland (1 359), United Kingdom (1 140), Netherlands (959), Sweden (924), Norway (907), Denmark (827), Belgium (689), Austria (655), Greece (228), Czech Republic (214), Finland (210), Slovenia (181), Portugal (169), Iceland (138), Ireland (129), Estonia (115), Romania (113), Poland (104), San Marino (92), Russia (59), Serbia (46), Slovakia (44), Bulgaria (41), Albania (38), Luxembourg (38), Croatia (37), Hungary (31), Georgia (30), Latvia (26), Belarus (21), Cyprus (21), Armenia (20), Moldova (20), Azerbaijan (19), Malta (18), North Macedonia (13), Lithuania (9), Liechtenstein (4), Bosnia and Herzegovina (3), Monaco (3), Ukraine (3), Andorra (2), Turkey (2) and Holy See (1).
Oceania: Australia (249) and New Zealand (8).
Other: International conveyance in Japan (696).
HOST AND RESERVOIR
Wild animal, bats [8] is the most possible host of the 2019-SARS-CoV-2. It requires further confirmation whether pneumonia infected by the 2019-SARS-CoV-2 is transmitted directly from bats or through an intermediate host. It is believed that clarifying the source of the virus will help determine zoonotic transmission patterns [12].
ROUTE OF TRANSMISSION
Up to present, the main infection source was the patients who with pneumonia infected by the 2019-SARS-CoV-2. Respiratory droplet transmission is the main route of transmission, and it can also be transmitted through contact [8]. Although many details, such as the source of the virus and its ability to spread between people remain unknown, an increasing number of cases show the signs of human-to-human transmission [9].
ETIOLOGY AND PATHOGENESIS
The 2019-SARS-CoV-2 isolated from the lower respiratory tract of patients with unexplainable pneumonia in Wuhan, and it is a novel coronavirus belonging to the β genus. The 2019-SARS-CoV-2 has an envelope; its particles are round or oval, often polymorphic, with a diameter from 60 nm to 140 nm. Its genetic characteristics are significantly different from SARSr-CoV (SARS related coronaviruses) and MERSr-CoV (MERS related coronaviruses). Current research shows it has more than 85% homology with SARSr-CoV (bat-SL-CoVZC45). 2019-SARS-CoV-2 can be found in human respiratory epithelial cells 96 h after in vitro isolation and culture, while it takes about 6 days in VeroE6 or Huh-7 cell lines [10].
The source of the virus, the time span of the patients discharging infective virus, and the pathogenesis are still not clear [11].
MOLECULAR EPIDEMIOLOGY
No evidence of viral mutation has been found so far [12]. It is necessary to obtain much more clinically isolated viruses with time and geographical variety to assess the extent of the virus mutations, and also whether these mutations indicate adaptability to human hosts [13].
INCUBATION AND CONTAGIOUS PERIOD
Based on currently epidemiological survey, the latency period is generally from 3 to 7 days, with a maximum of 14 days [13]. Unlike SARSr-CoV, 2019-SARS-CoV-2 is contagious during the latency period [14].
PROGNOSTIC FACTORS
The population is generally susceptible to the virus. The elderly and those with underlying diseases show more serious conditions after infection, and children and infants also get infected by the 2019-SARS-CoV-2. From current knowledge of the cases, most patients have a good prognosis, the symptoms of children are relatively mild, and a few patients are in critical condition. Death cases are more frequently seen in the elderly and those with chronic underlying diseases [15].
The newest study including the first 41 confirmed cases admitted to Wuhan between 16 December 2019 and 2 January 2020 showed the median age of patients was 49 years; and the main underlying diseases were diabetes, hypertension, and cardiovascular diseases. Of them, 12 cases experienced acute respiratory distress syndrome (ARDS), 13 cases were admitted to the intensive care unit (ICU), and 6 cases died [16].
DIAGNOSIS OF THE 2019-SARS-COV-2 CASES
CLINICAL MANIFESTATION
The 2019-SARS-CoV-2 infected cases have symptoms like fever, fatigue, dry cough, dyspnea etc., with or without nasal congestion, runny nose or other upper respiratory symptoms [17, 18]. Despite the atypical symptoms were reported [19], Nan-Shan Zhong, the academician of Chinese Academy of Engineering in an exclusive interview with Xinhua News Agency on 28 January 2020, pointed out that fever is still the typical symptom of 2019-SARS-CoV-2 infection.
PHYSICAL EXAMINATION [20]
Patients with mild symptoms may not present positive signs. Patients in severe condition may have shortness of breath, moist rales in lungs, weakened breath sounds, dullness in percussion, and increased or decreased tactile speech tremor, etc.
IMAGING EXAMINATION: CT IMAGIN (STRONG RECOMMENDATION) [21]
The imaging findings vary with the patient's age, immunity status, disease stage at the time of scanning, underlying diseases, and drug interventions.
The imaging features of lesions show: (1) dominant distribution (mainly subpleural, along the bronchial vascular bundles); (2) quantity (often more than three or more lesions, occasional single or double lesions); (3) shape (patchy, large block, nodular, lumpy, honeycomb-like or grid-like, cord-like, etc.); (4) density (mostly uneven, a paving stones-like change mixed with ground glass density and interlobular septal thickening, consolidation and thickened bronchial wall, etc.); and (5) concomitant signs vary (air-bronchogram, rare pleural effusion and mediastinal lymph nodes enlargement, etc.).
CLINICAL DATA FROM ZHONGNAN HOSPITAL OF WUHAN UNIVERSITY [22]
Typical CT/X-ray imaging manifestation, include:
Multiple, patchy, sub-segmental or segmental ground-glass density shadows in both lungs. They were classified as “paving stone-like” changes by fine-grid or small honeycomb-like thickening of interlobular septa. The thinner the CT scan layers, the clearer the ground-glass opacity and thickening of interlobular septa were displayed. A slightly high-density and ground-glass change with fuzzy edge in the fine-grid or small honeycomb-like thickening of interlobular septa were presented by the high-resolution computed tomography (HRCT), (Figure 1: 45 cases, 54.2% in a total of 83 cases). The resolution of X-ray was worse lower than that of CT in the resolution, which was basically manifested as ground-glass opacities with fuzzy edge (Figure 2: 9 cases, 10.8% in a total of 83 cases).
Multiple, patchy or large patches of consolidation in both lungs, with a little grid-like or honeycomb-shaped interlobular septal thickening, especially in the middle and lower lobes (Figure 3: 26 cases, 31.3% in a total of 83 cases). It was more common in the elderly or severe condition patients.
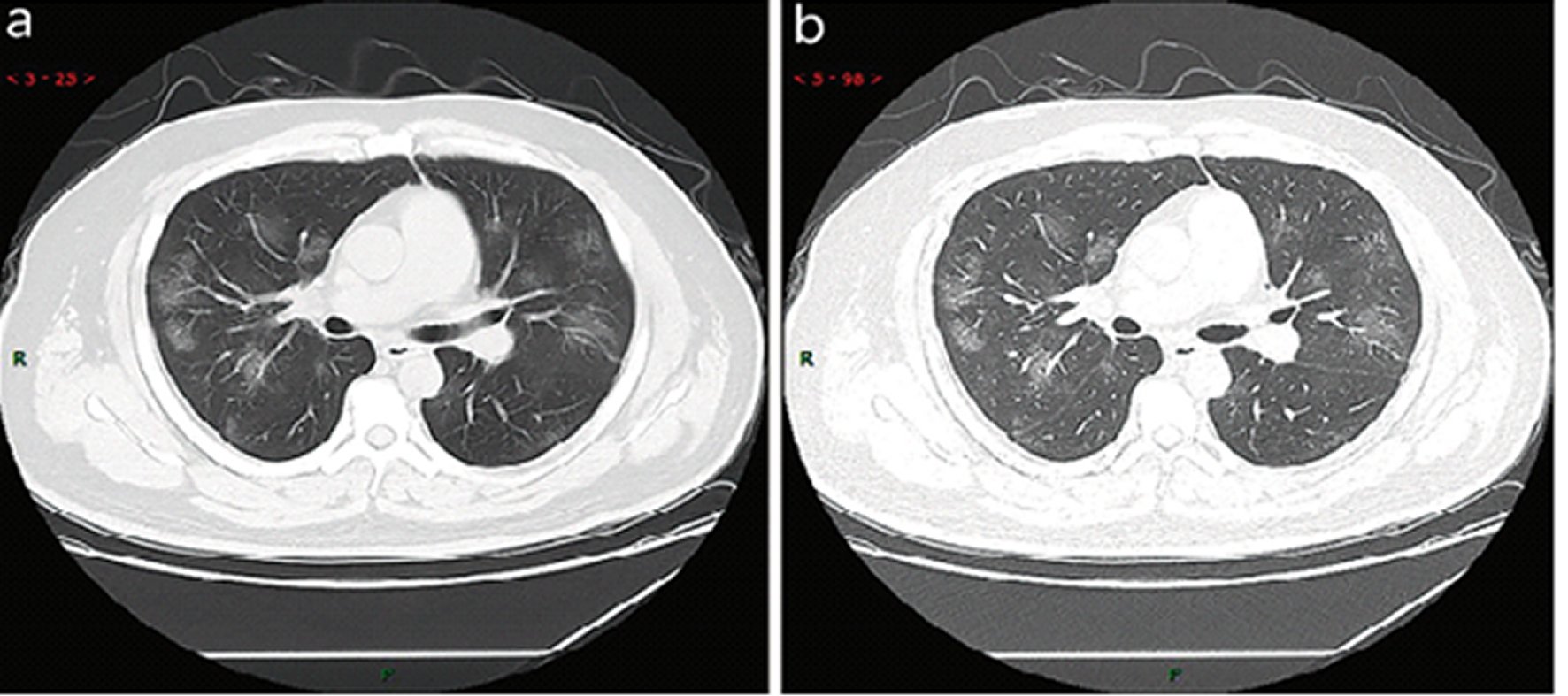
Figure 1. Typical CT imaging manifestation (case 1). A 38 years old male with fever without obvious inducement (39.3 °C), dry cough and shortness of breath for 3 days. Laboratory test: normal white blood cells (6.35 × 109/L), decreased lymphocytes percentage (4.1%), decreased lymphocytes count (0.31 × 109/L), decreased eosinophil count (0 × 109/L)), increased C-reaction protein (170.91 mg/L), increased procalcitonin (0.45 ng/ml). Imaging examination: multiple patches, grid-like lobule and thickening of interlobular septa, typical “paving stone-like” signs. a SL(Slice): 6 mm; b high-resolution computed tomography (HRCT). HRCT. high-resolution computed tomography.
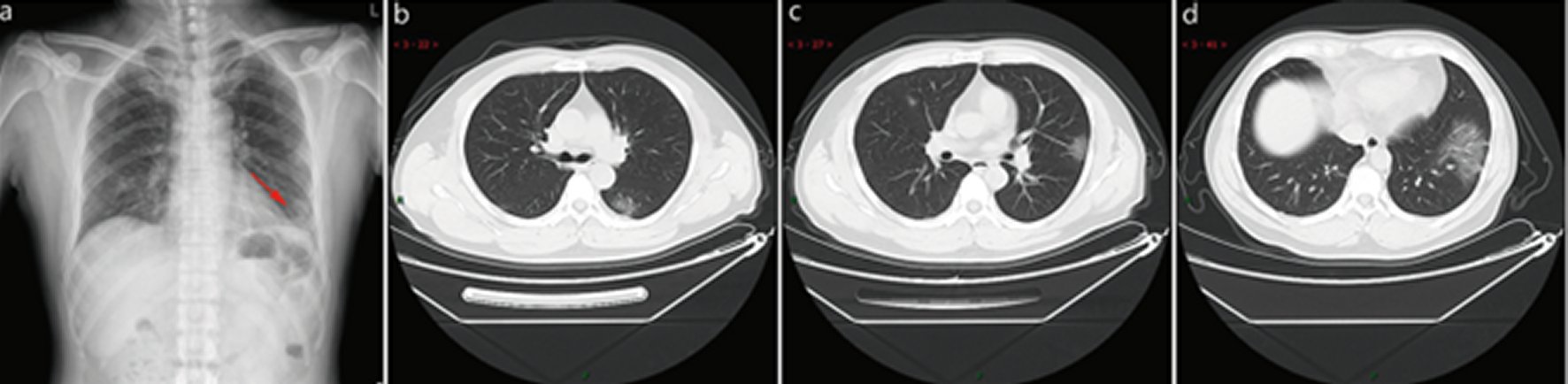
Figure 2. Typical CT / X-ray imaging manifestation (case 2).A 51 years old male with general muscle ache and fatigue for 1 week, fever for 1 day (39.1 ℃), anemia. Laboratory test: normal white blood cells (9.24 × 109/L), lymphocytes percentage (5.1%), decreased lymphocytes (0.47 × 109/ L), decreased eosinophil count (0 × 109/L), increased C-reaction protein (170.91 mg/L), increased procalcitonin (0.45 ng/ml), increased erythrocyte sedimentation rate (48 mm/h). Imaging examination: a shows patchy shadows in the outer region of the left lower lobe, b shows large ground-glass opacity in the left lower lobe, and c shows subpleural patchy ground-glass opacity in posterior part of right upper lobe and lower tongue of left upper lobe, d shows large ground-glass. opacity in the basal segment of the left lower lobe.

Figure 3. Typical CT / X-ray imaging manifestation (case 3).A 65 years old male with fever for 4 days (38.7 ℃). Laboratory test: normal white blood cells (3.72 × 109/L), decreased lymphocytes (0.9 × 109/ L), decreased eosinophil count (0 × 109/L), increased C-reaction protein (53.0 mg/L), increased procalcitonin (0.10 ng/ml), reduced liver function, hypoproteinemia, and mild anemia. Imaging examination: a and b showed large consolidation in the right middle lobe, patchy consolidation in the posterior and basal segment of the right lower lobe, with air-bronchogram inside, c showed patchy consolidation in the outer and basal segment of the left lower lobe, and a small amount of effusion in the right chest.
Atypical CT/X-ray imaging manifestation, include:
Single, or multiple, or extensive subpleural grid-like or honeycomb-like thickening of interlobular septum, thickening of the bronchial wall, and tortuous and thick strand-like opacity. Several patchy consolidations, occasionally with a small amount pleural effusion or enlargement of mediastinal lymph nodes, can be seen (Figure 4: 6 cases, 7.2% in a total of 83 cases). This is mostly seen in the elderly.
Single or multiple solid nodules or consolidated nodules in the center of lobule, surrounded by ground-glass opacities (Figure 5: 5 cases, 6.2% in a total of 83 cases).
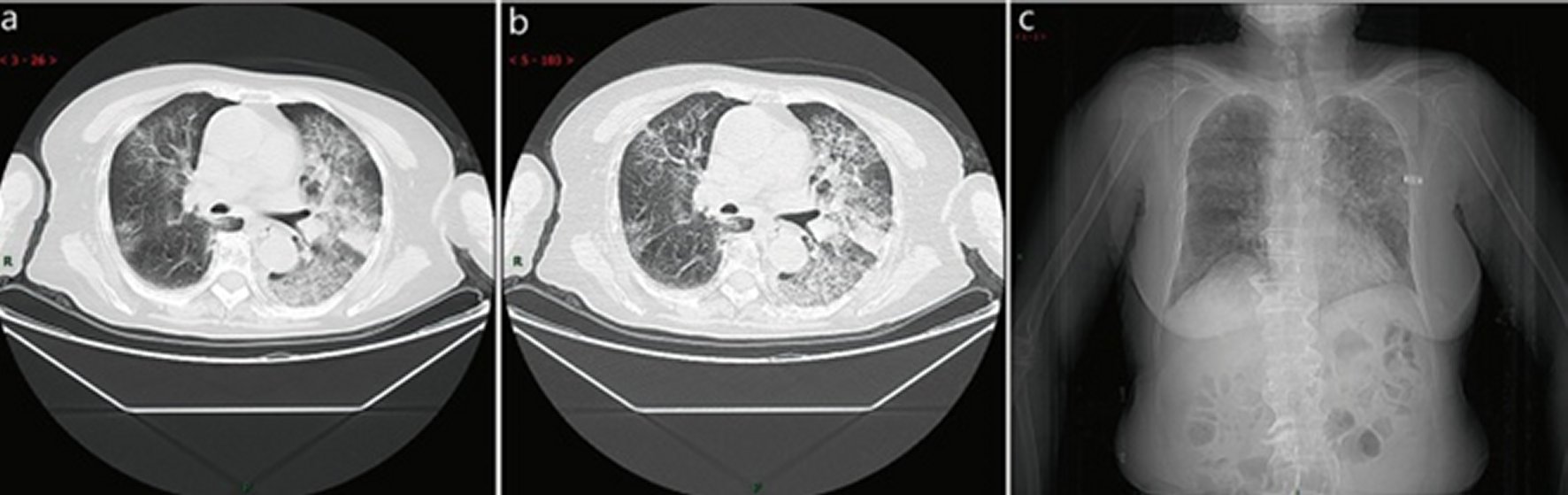
Figure 4. Atypical CT / X-ray imaging manifestation (case 1).An 83 years old female with fever for 4 days (maximum temperature of 38.8 ℃), cough, chills, sore throat, dry cough for 1 week, chest tightness and shortness of breath aggravating for 1 week. Laboratory test: normal white blood cells (4.6 × 109/L), normal neutrophil percentage (65.8%), decreased lymphocytes percentage (19.9%). Imaging examination: a and b showed diffuse interlobular septum thickening in both lungs to form a grid opacity, thickening of bronchial wall, and consolidation in the left sublobal lung. c showed diffused grid-like opacities in both lungs, especially in the left lung opacity in the basal segment of the left lower lobe.
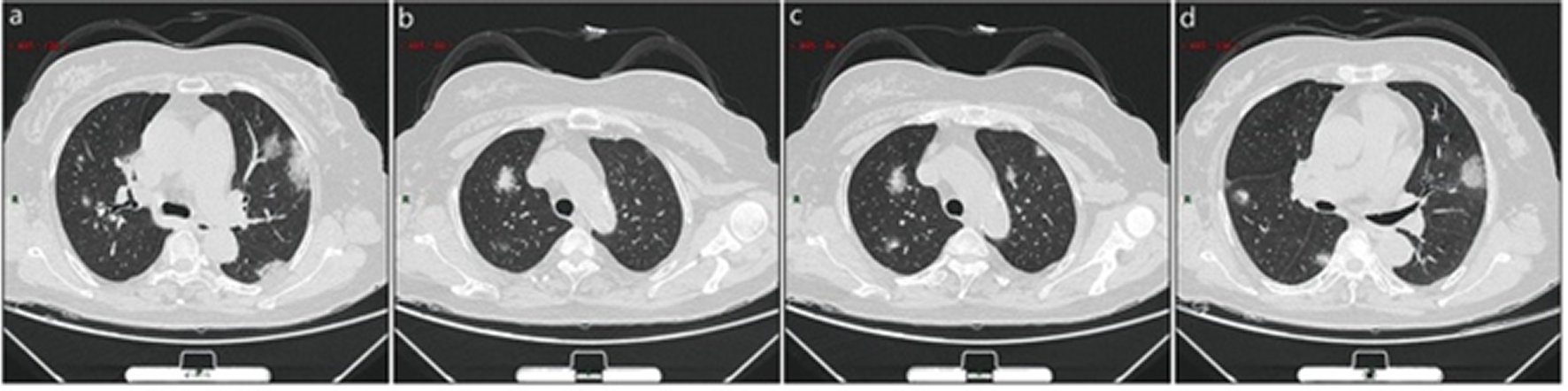
Figure 5. Atypical CT / X-ray imaging manifestation (case 2).A 56 years old female with fever for 3 days. Laboratory test: decreased total protein (54.0 g/L),decreased albumin (35.5 g/L),decreased globulin (18.5 g/L), normal white blood cells (4.87 × 109/L), decreased lymphocytes percentage (10.1%), decreased lymphocytes (0.49 × 109/ L), decreased eosinophil count (0 × 109/L)), decreased eosinophil count percentage (0%). Imaging examination: a showed two consolidation nodulesat the center of the lateral segment of middle lobe of the right lung which was surrounded by ground-glass opacities; b showed patchy ground-glass opacity in the anterior segment of the right upper lung with patchy consolidation lesions in it; c showed patchy ground-glass opacities in both lungs with patchy consolidation lesions in it. d showed patchy consolidation in the ground-glass opacities in the middle lobe and dorsal segment of lower lobe of right lung.
Stage based on CT image [24]:
The CT imaging demonstrates 5 stages according to the time of onset and the response of body to the virus, including:
Ultra-early stage. This stage usually refers to the stage of patients without clinical manifestation, negative laboratory test but positive throat swab for 2019-SARS-CoV-2 within 1–2 weeks after being exposed to a virus-contaminated environment (history of contact with a patient or patient-related family members, unit, or medical staff in a cluster environment). The main imaging manifestations are single, double or scattered focal ground-glass opacity, nodules located in central lobule surrounded by patchy ground-glass opacities, patchy consolidation and sign of intra-bronchial air-bronchogram, which was dominant in the middle and lower pleura (Figure 6: 7 cases, 8.4% in a total of 83 cases).
Early stage. This stage refers to the period of 1–3 days after clinical manifestations (fever, cough, dry cough, etc.). The pathological process during this stage is dilatation and congestion of alveolar septal capillary, exudation of fluid in alveolar cavity and interlobular interstitial edema. It showed that single or multiple scattered patchy or agglomerated ground-glass opacities, separated by honeycomb-like or grid-like thickened of interlobular septa (Figure 7: 45 cases, 54.2% in a total of 83 cases).
Rapid progression stage. This stage refers to the period about 3–7 days after clinical manifestations started, the pathological features in this stage are the accumulation of a large number of cell-rich exudates in the alveolar cavity, vascular expansion and exudation in the interstitium, both of which lead to further aggravation of alveolar and Interstitial edema. The fibrous exudation connects each alveolus through the inter-alveolar space to form a fusion state. The CT manifested a fused and large-scale light consolidation with air-bronchogram inside (Figure 8: 17 cases, 20.5% in a total of 83 cases).
Consolidation stage. This stage refers to the period around 7–14 days after clinical manifestations appeared. The main pathological features in this stage are the fibrous exudation of the alveolar cavity and the disappearance of capillary congestion in the alveolar wall. CT imaging showed the multiple patchy consolidations in slighter density and smaller range than that of the previous stage. (Figure 9: 26 cases, 31.2% in a total of 83 cases).
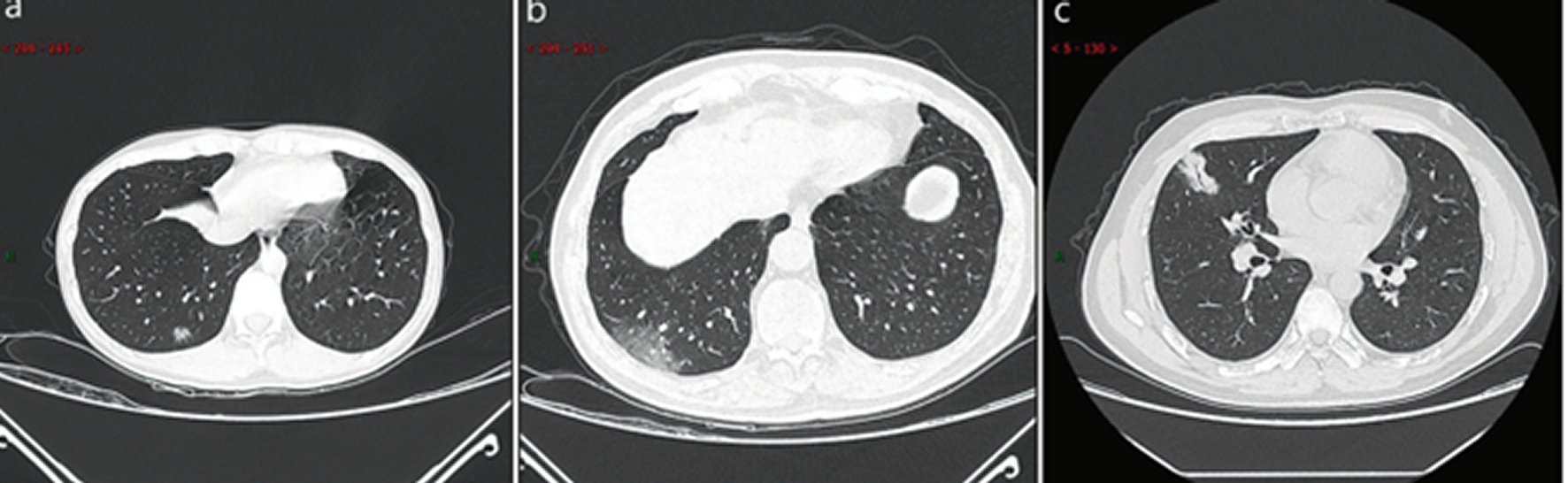
Figure 6. CT imaging of ultra-early stage.a: A 33 years old female with patchy ground-glass opacities after occupational exposure. b: A 67 years old male with a history of contact with infected patients, showing large ground-glass opacity. c: A 35 years old female exhibiting large consolidated opacity with air-bronchogram inside after occupational exposure.
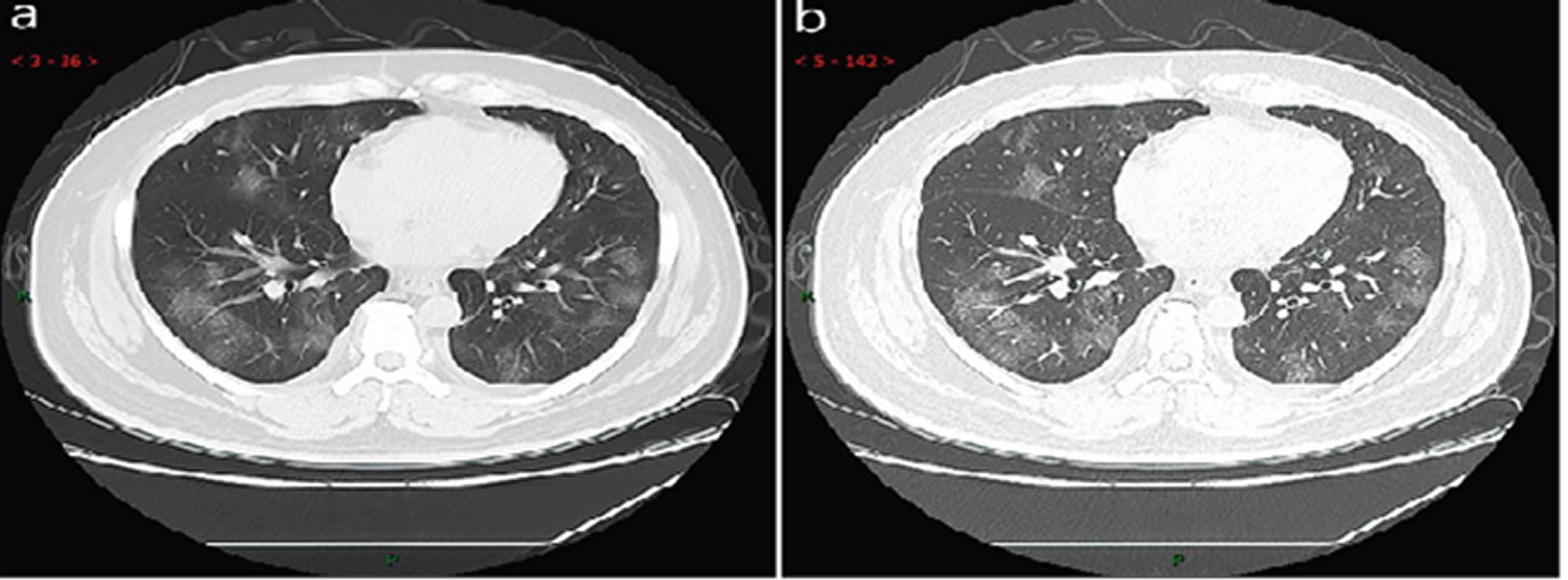
Figure 7. CT imaging of early stageMale, 38 years old, fever without obvious inducement (39.3 ℃), dry cough and shortness of breath for 3 days. Laboratory test: decreased white blood cells (3.01 × 109/L), decreased lymphocytes (0.81 × 109/ L), increased C-reaction protein (60.8 mg/L), increased procalcitonin (0.16 ng/ml). Imaging examination: a (thin layer CT) and b (high-resolution CT) showed multiple patchy and light consolidation in both lungs and grid-like thickness of interlobular septa.
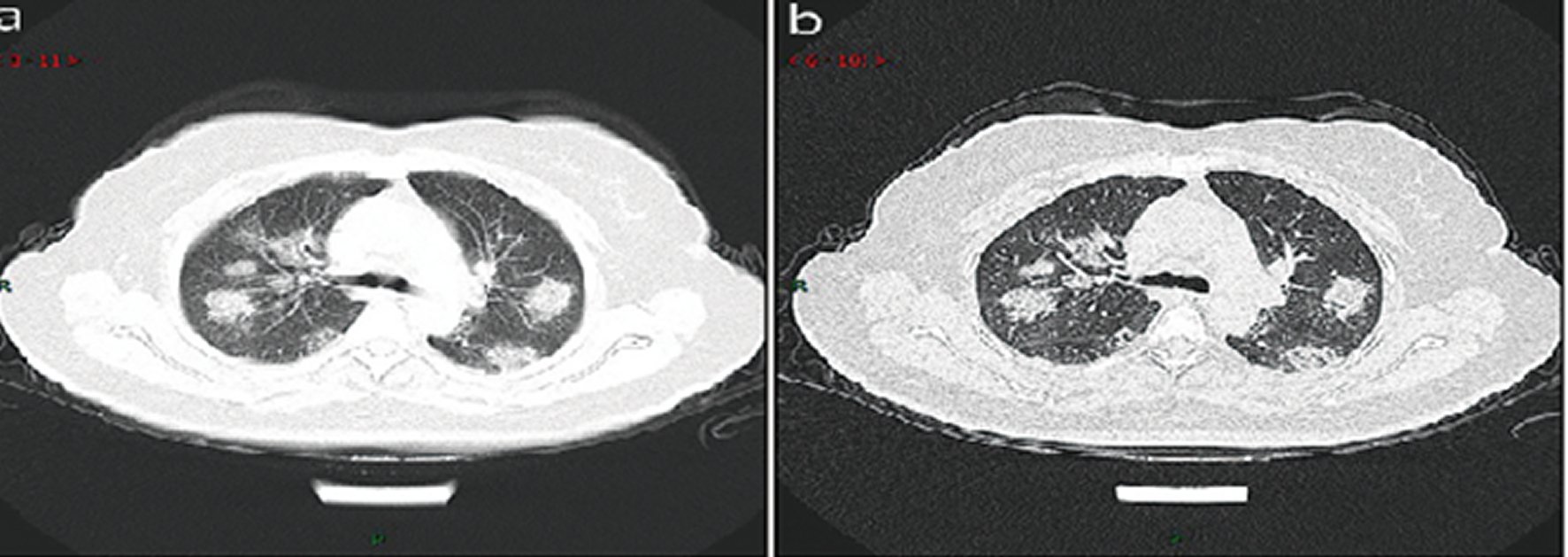
Figure 8. CT imaging of rapid progression stage.A 50 years old female with anorexia, fatigue, muscle soreness, nasal congestion and runny nose for 1 week, sore and itching throat for 2 days. Laboratory test: increased erythrocyte sedimentation rate (25 mm/h), normal white blood cells (4.08 × 109/L), decreased lymphocytes (0.96 × 109/ L), increased C-reaction protein (60.8 mg/L). Imaging examination: a (thin layer CT) and b (high-resolution CT) showed multiplepatchy and light consolidation in both lungs and grid-like thickness of interlobular septa.
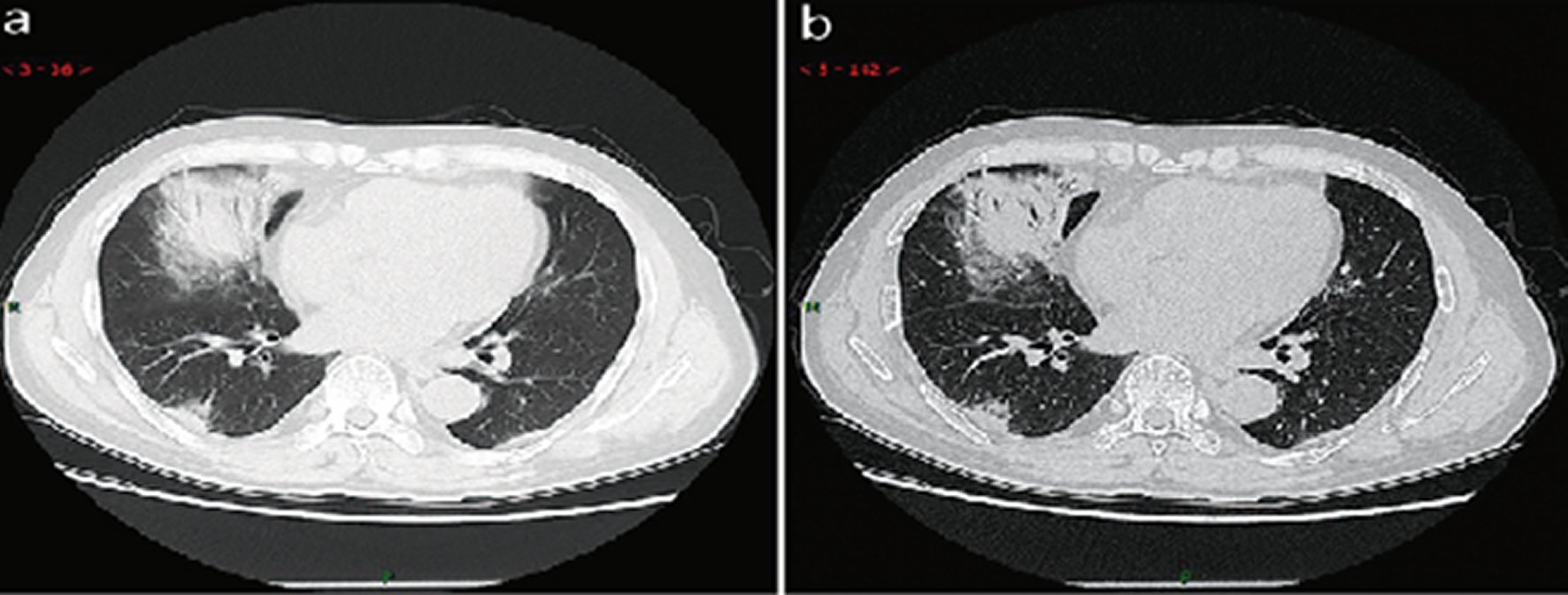
Figure 9. CT imaging of consolidation stage.A 65 years old male with fever (maximum temperature of 39 ℃). Laboratory test: hypoproteinemia (decreased total protein (62.20 g/L), decreased albumin (35.70 g/L)), abnormal liver function (increased alanine aminotransferase (79 U/L), increased aspartate aminotransferase (72 U/L)), increased procalcitonin (0.10 ng/ml), increased C-reaction protein (53 mg/L), decreased white blood cells (3.72 × 109/L), decreased lymphocytes (0.9 × 109/ L), mildanemia (decreased red blood cells (4.10 × 1012/L), decreased hemoglobin (131.10 g/L), decreased hematocrit (39.0%). Imaging examination: a (thin layer CT) and b (high-resolution CT) showedmultiple patchyand large consolidation in right middle lobe, posterior and basal segment of right lower lobe and outer and basal segment of left lower lobe, with air-bronchogram inside.















