INTRODUCTION
Gastroschisis (GS) is defined as a congenital anterior abdominal wall defect characterized by the evisceration of intra-abdominal organs without covering membrane through a weakness in the form of cleft on the right side of umbilical cord [1, 2].
GS is otherwise referred to as "cleft belly" in the Ancient Greek language that was first described by Calder in the 16th century in the medical literature [1, 2]. Some authors submitted that there were no reports of survivors until Watkins closed the defect on a baby with GS in 1943 [1, 2]. In 1953 Moore and Strokes laid to rest the distinction between omphalocele and GS as separate pathological entities [1, 3]. Interestingly, Duhamel [1, 4, 5] in 1963 further elaborated on their different pathogenesis and clinical manifestations. In cases with GS, the localization and development of the umbilicus remain normal. However, due to the failure of the right omphalomesenteric artery, a full-thickness anterior abdominal wall defect arises in this area. Thus abdominal organs herniate through this defect [1, 4, 5].
Meanwhile, abdominal wall defects are rare anomalies. GS is relatively typical, with an estimated frequency of occurrence at 1/4000 to 4 - 5/10000 live births [6, 7, 8]. Another author argues that there is a high prevalence rate of GS in babies delivered by relatively young mothers [6, 9]. Even though the mortality figures from GS have significantly reduced over the years, from 90% to 10%, morbidity remains high, and GS continues to provide a challenge to pediatric surgeons worldwide [6, 10, 11, 12]. Since membranes do not cover GS, the eviscerated structures are exposed to amniotic fluid and other external substances after birth, which increases the risk of infection and injuries [6, 13, 14, 15, 16]. Some authors submitted that GH is a low-prevalence disease [6, 17] of great importance due to the excellent prognosis and survival of patients [6, 18, 19], especially in the clinical scenario where prompt intervention and adequate management is provided [6, 20, 21].
Furthermore, there is typically yet no consensus as per the different techniques of GS closure emanating from multiple reports. These options include (1) primary closure with umbilical preservation [21, 22], (2) elective delayed midgut reduction without anesthesia, as described by Bianchi and Dickson [23, 24], (3) delayed repair with a preformed silo [25, 26].
We report a case of GS seen in our center, highlighting the rarity of this clinical entity. The author equally reviewed the existing literature covering the epidemiology, etiopathogenesis, management options, and outcomes of GS in low-income countries (LIC) in SSA and compares the reports with middle- (MIC) and high-income countries (HIC) across the world.
CASE PRESENTATION
We report a female newborn, daughter of a 26- year-old mother, who attended three antenatal checkups at the primary health center in Kambe, a rural setting within the northwestern region of Cameroon. Interestingly, she started her prenatal program at the third trimester of pregnancy, all attentions were given by a primary care nurse, and an obstetric ultrasound was not performed nor indicated during the pregnancy. According to the antenatal card, adequate weight growth was reported. Besides, the personal history of the parents did not show exposure to toxic substances or X-rays. There are no reports in the antenatal period of any urinary tract infection or any pathology during the pregnancy. Both parents deny a history of omphalocele, GS, or other congenital diseases in the family tree and deny consanguinity. This GS is the mother's fifth pregnancy, no previous abortions, and no death or inherent complications on the other four children.
The pregnancy was pre-term, and delivery was at 36 completed weeks, performed by her care nurse at the same primary health center in Kambe, and was subsequently transferred to our neonatology service. The index baby was delivered by spontaneous vaginal delivery with an incidental finding of protruding, violaceous, and wet intestinal loops, associated with respiratory distress. The patient received oxygen therapy through nasal prongs, while initial ophthalmic prophylaxis was performed.
Furthermore, the neonate only succeeded in reaching our tertiary care facility following referral to us after about six hours post-delivery. Subsequently, immediate gastric lavage was performed, and some saturated saline-soaked sterile gauze was placed, and antibiotic treatment with ampicillin-gentamicin was initiated. Ringer's lactate solution and 10% dextrose in the water at 100mls/kg/day were administered with a metabolic flow of 6.7 mg/kg/min, and inotropic management was initiated due to hemodynamic instability.
In addition to the above, the child was the fifth of her mother with O positive blood type, who underwent five antenatal care checkups, serology, and protocol blood tests with negative results. Obstetric ultrasounds at 30weeks of pregnancy did not report alterations, and fetal kicks were positive since the gestational age of two months. The infant was a vaginal delivery product with cephalic presentation and without premature rupture of membranes; Apgar: 6/8/10. The child was fully vaccinated. No pathological, infectious, pharmacological, or transfusion history was observed other than maternal consumption of local herbal concoction during the first trimester of pregnancy. She admitted to having taken a significant amount of local herbal mixture at first trimester for the treatment of a febrile illness. Besides, she observed slight jaundice and pedal swelling during the same period; both symptoms resolved spontaneously without consultation with a medical practitioner. The physical examination revealed stable vital signs and standard anthropometric measurements. The birth weight estimate was 2.45kg (abdominal Perimeter was not assessed due to the protrusion of intestinal loops). The thorax showed a slight intercostal retraction and the abdomen; a protrusion of stomach and intestinal loops were pink, covered with saline-soaked wet gauze, and producing a foul odor. The skin was pale and poorly perfused.
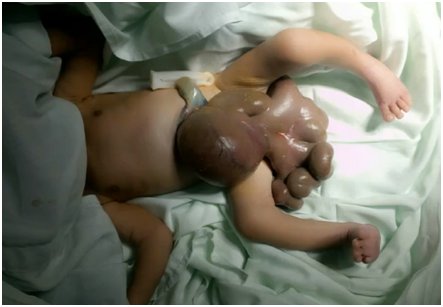
Based on the above clinical findings, GS, respiratory distress syndrome, and early neonatal sepsis were diagnosed. The renal ultrasound, whole-body radiographs, and echocardiogram did not show any associated congenital malformations; the results were typical. Clinical genetics suggested that a chemical teratogenic disruptive process during the first trimester of pregnancy was the probable etiology for the GS. Also, the patient should have benefitted from admission to the Neonatal intensive care unit (NICU) for mechanical ventilation and inotropic support. However, a mechanical ventilator and experienced pediatric anesthesiologist were not available. The Pediatric Surgery Unit scheduled closing the abdominal wall gradually and adding metronidazole to antibiotic management. During surgery, severe GS was found with exposure of stomach, small and large intestines, intestinal malrotation with thickened edematous mesentery, and leaky and thickened intestine due to intrauterine exposure as seen in Figure 1.
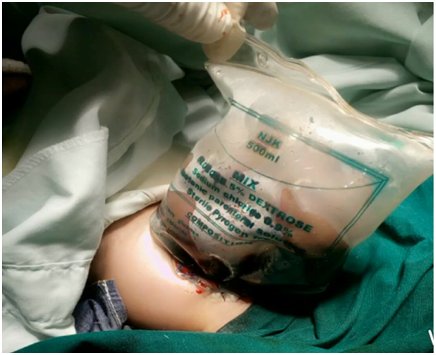
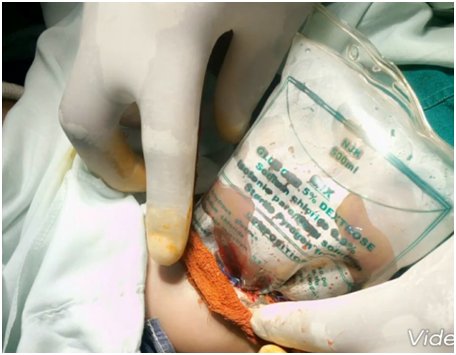
The umbilical border was cleared, the umbilical and vesical arteries were ligated, and an infusion drip bag as improvised silo was attached to the skin covered with gauze impregnated with betadine as seen in Figure 2 and Figure 3. The procedure was well tolerated at first, but a deterioration of the clinical condition was observed. Subsequently, with hemodynamic instability, the patient obviously will require further inotropic support with dopamine and dobutamine, and endotracheal re-intubation with manual ventilation by Ambu-bagging. Nonetheless, sedation and skeletal muscle relaxant were not administered due to the patient's poor clinical status. Moreover, the patient was followed up with antibiotic therapy with ampicillin-gentamicin and metronidazole. Consequentially, the patient clinical condition continued to deteriorate until the third postoperative day when she went into cardiopulmonary arrest and was eventually certified dead.
DISCUSSION
EPIDEMIOLOGY
Several literature reports submitted that there had been an unprecedented rise in the incidence of GS worldwide, especially in the last three decades [27, 28]. Interestingly, the situation is indifferent, especially in the low-income and middle-income countries (LMICs), because the overall incidence is generally increasing [27, 28]. Also, there is a rise in the number of cases presenting to a healthcare facility. For instance, an estimated 35-fold surge in cases was reported in Pretoria, South Africa, between 1981 and 2001 [27, 28].
The adjudged reason for such an increase in the prevalence could not be substantiated. A few authors suggested increasing prenatal ultrasound use, which makes the clinician be able to pick up the at-risk fetus very early [29, 30]. Meanwhile, a few cases were seen in Southern America, covering Honduras, Chile, Brazil, and then Australia, etc. [29, 30]. One literature reported that there had been an estimated 300% increase in the incidence of GS from 1994 up till 2015, otherwise regarded as a “Gastroschisis pandemic which is strongly related to low maternal age that is maternal age <20 years” [29, 30]. There is equally an association between race and complex GS. Besides, the low socioeconomic status of parents is closely related to mortality in such babies [29, 30]. There has been a report of an estimated 30% increase in incidence in black-skinned neonates and mixed neonates [29, 31]. This association is in concordance with our patient, daughter of a black African father and mother, with low socioeconomic statuses, that conceived a black African newborn with complex GS. In addition to multiple congenital malformations and did not survive beyond a few days of life [29, 31].
Moreover, other risk factors associated with the disease are i) prematurity; ii) small for gestational age newborns; iii) being born to a primigravida mother; iv) Caucasian race; v) Hispanic mothers; vi) maternal malnutrition; vii) exposure to nitrosamines; viii) teratogens and agrochemicals; ix) consumption of non-steroidal anti-inflammatory drugs and acetaminophen in the first trimester; x) cigarette, alcohol and illicit drugs consumption; xi) absence of prenatal checkups and short cohabitation with the father of the child [17, 18, 19, 20, 21]. The average age of mothers with affected children is 21.1 years; women aged 14 to 19 have a 7.2 times higher risk of having a child with GS compared to 25 to 29-year-old mothers [13, 32]. It should be noted that the mother of the studied patient was 26 years old, multigravida, exposed to a toxic substance (herbal concoction) in the first trimester of pregnancy, and low socioeconomic status.
ETIPATHOGENESIS
Available literature reports submitted that scientists are yet to establish the etiological factor for GS. However, the condition is considered a multifactorial disease [13, 21]. Embryologically, the abdominal wall originates from the lateral mesoderm and by the fusion of four folds (cephalic, caudal, and two lateral foldings), which grow towards the midline, converging in the umbilical ring that is completed around the fourth week [13, 21]. Evidence-based studies support the current causal theories, which affirm that vascular disruptions cause GS [13, 33, 34, 35]. Typically, the vascular accident could be due to a) intrauterine occlusion of the omphalomesenteric artery, or b) early atrophy (<28 days) of the right umbilical vein; which causes wall infarction with rupture of the umbilical ring and eventration of the intestine [13, 33, 34, 35]. Another new theory proposes a fault in the yolk sac during embryonic development, with the consequent formation of an additional opening through which the intestine is eventuated, instead of doing it through the umbilical cord [33, 34, 35]. The outcome is the eventration of abdominal contents in utero that, regardless of the size and quantity of viscera exposed, is associated with a mortality of 5% and 3-15% after birth [7, 8, 9, 10, 11, 12]. However, these deaths may be related to complications and significant morbidity; and prolonged hospital stays, need for mechanical ventilation, prolonged parenteral nutrition, multiple surgical interventions, and diseases such as intestinal atresia, short bowel syndrome, neonatal sepsis and necrotizing enterocolitis [7, 8, 9, 10, 11, 12].
CLINICAL PRESENTATIONS
The typical anatomical presentation is that of a defect on the anterior abdominal wall; usually, about 2-4 cm sized full-thickness abdominal wall [1, 2, 3, 4]. The fault is located mostly on the right, rarely left lateral side of the umbilical cord, while the location of the umbilical cord remains unchanged [1, 2, 3, 4], as seen in Figure 1. Frequently, intestines and sometimes stomach, colon herniates towards the outside of the abdomen. Besides, in some clinical scenarios, bladder, uterus, tubes, even testicles, and ovaries may also eviscerate thorough the defect. If the liver occasionally is located outside of the fault, this indicates a poor prognosis [1, 2, 3, 4]. On display in Figure 1 is the complete gastric herniation and bowel evisceration in the index patient.
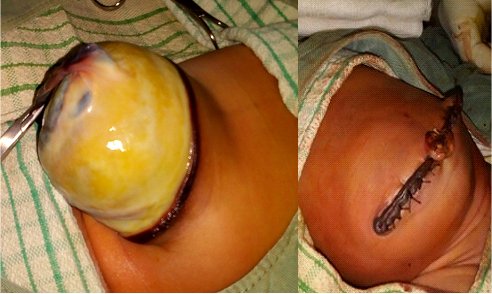
The membrane does not cover the organs herniating outside. Due to this fact and the chemical effect of amniotic fluid, the intestines are inflamed. The quality of the pipes is the primary factor, which determines the prognosis of a patient with GS [1, 2, 3, 4]. The intestines become thickened due to prolonged contact with amniotic fluid; sometimes, they even could contain calcifications [1, 2, 3, 4]. Detection of calcification could lead to the consideration of intestinal perforation. Observing the expanding abdominal diameter and the increase of wall thickness in the USG examination demonstrates intestinal damage. Polyhydramnios could be considered if intestinal obstruction occurs [1, 2, 3, 4]. The classical differences between gastroschisis and omphalocele are outlined in Table 1, while a giant omphalocele is displayed in Figure 4.
DIAGNOSIS
One primary technique contributing to the prenatal diagnosis of GS is to determine alpha-fetoprotein (AFP) and amniotic-fluid acetylcholinesterase levels in the mother's blood. Fetal ultrasonography and magnetic resonance imaging bring significant benefits to diagnosis [1, 2, 3, 4]. The fact that AFP levels could also rise in fetal anomalies such as Spinal Bifida should always be considered as the possible co-existing condition [1, 2, 3, 4]. Therefore, it is recommended to determine the acetylcholinesterase/pseudocholinesterase ratio in amniotic fluid if encountered with these situations. Due to prevailing resource constraints, such prenatal diagnostic techniques could not be done at our facility, as noted in the index patient [1, 2, 3, 4].
Valid ultrasonography results could only be achieved after the 14th-17th gestational week. The ultrasonography image of GS is seen as a small anterior abdominal wall defect located right lateral side of the umbilical cord and intestines dangling from this area to amnion space [1, 2, 3, 4]. Colored Doppler Ultrasound could be used to demonstrate regular umbilical cord access [1, 2, 3, 4]. The safest transport of newborns is done in the mother's abdomen. That is why fetuses that are expected to be born at risk should be delivered in centers providing newborn intensive care units [1, 2, 3, 4].
Furthermore, diagnosis facilitates better monitoring of pregnancy, which avoids complications [13, 36, 37]. There are useful ultrasound predictors to estimate the possibility of neonatal complications, such as intestinal atresia [13, 17, 37]. Some predictors are intra-abdominal dilation of the bowel, intrauterine growth restriction, the thickness of the abdominal wall, and liver herniation [13, 17, 18].
MANAGEMENT
The current practice and guidelines suggest that for successful gastroschisis management the following must be in place including a) Prenatal diagnosis, b) Delivery in a tertiary pediatric surgery center, c) Adequate pre-hospital management and transfer, d) Pre-intervention resuscitation, e) Bowel reduction and defect closure, f) Post-intervention neonatal care, and g) Provision of parenteral nutrition (PN) until enteral feeding is established, and the revered multidisciplinary team approach [38, 39].
There is no consensus about delivering babies antenatal diagnosed with GS by planned C-section [1, 2, 3, 4, 5, 6]. Furthermore, providing the baby by C-section does not affect the prognosis significantly except the reduction of the risk of intra-abdominal organ trauma and infection [1, 2, 3, 4, 5, 6]. A common challenge in those with C-section is predating the birth to a very early date does not bring significant benefit; instead, it causes problems related to prematurity. Interestingly, even in cases where there are no problems associated with fetal distress or intestinal damage, delivering the baby is around 37th week could be relevant [1, 2, 3, 4, 5, 6]. The transfer of the baby to a center in time providing antenatal, obstetric, neonatal, and pediatric surgery care coordinately remains a critical issue in the management of the disease [1, 2, 3, 4, 5, 6]. For such babies born with GS, measures that must be taken include adequate life support given immediately after birth to reduce mortality and morbidity significantly. Following the birth of the baby, the segments of the intestines located outside of the abdomen should be covered by sterile wet gauze bandage or plastic film. Thus baby should be kept warm [1, 2, 3, 4, 5, 6].
Meanwhile, fluid and electrolyte replacement should be applied. Due to the localization of the intestines outside the ventral cavity, these babies are in the tendency to lose their body fluids, and their body temperature tends to be lowered quickly [1, 2, 3, 4, 5, 6]. To provide adequate urine output and maintain the acid-base equilibrium, fluid a few times more than the requisite amount for an ordinary newborn could be necessary to replace into the babies with GS [150-200 ml/kg] [1, 2, 3, 4, 5, 6].
In most high-income countries (HICs), there exist wide variations in approach to bowel reduction and defect closure [22, 23, 24, 25, 26, 27]. Nonetheless, the two most commonly utilized techniques are primary closure in an operating room (OR) or cotside application of a preformed silo (PFS) with serial reductions over several days followed by cotside sutureless closure or closure in the OR [22, 23, 24, 25, 26, 27].
Treatment modalities include primary closure, establishing a ventral hernia to be closed later. Interestingly, the immediate covering of the eviscerating intestines and organs with silastic silo is preferred recently, as seen in our index patient (Figure 2 and Figure 3) [40, 41]. The prognosis is considered as good because of the frequency of having associated anomaly is low among the babies with GS. The mortality rate is known as between 5%-15%, mean 7.7%, provided respiratory and circulatory insufficiency, sepsis, or complications regarding total parenteral nutrition were not encountered [3, 4, 5, 6, 40, 41, 42]. The survival rate is stated as 96% in cases with isolated GS treated in well-equipped centers [40, 41].
Consequently, babies with GS represent an improved prognosis, parallel to advances in medicine in recent years. We believe that terminating these pregnancies is not a necessity. Therefore, diagnosing these babies in their prenatal, early periods, and transferring them to an experienced medical center containing multidisciplinary working facilities will contribute to the health of both the mother and the baby [40, 41].
CONCLUSION
The exact etiological factors for GS are yet to be established, and the condition is considered a multifactorial disease. More frequently than ever, it’s becoming easy to make prenatal diagnosis utilizing ultrasonography with high yield. GS continues to provide a challenge for pediatric surgeons worldwide. Nonetheless, it remains a low-prevalence disease with a very good prognosis, if initial management is adequate. Interestingly, the disease requires adequate knowledge from both specialized and primary care personnel. Once the clinicians are well equipped, they can readily ensure correct initial management. Besides, the patient can equally benefit from an appropriate and timely referral to tertiary care centers to avoid future complications.