INTRODUCTION
The emergence of novel coronavirus known as Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2), has presented an unprecedented challenge for the healthcare professionals across the world. High infectivity, ability to get transmitted even during asymptomatic phase and relatively low virulence has resulted in rapid transmission of this virus beyond geographic regions, leading to a pandemic. The first case of this disease, known as coronavirus disease 2019 (COVID-2019), occurred on December 8, 2019 in the Hubei province of China [1]. Since then, the statistics are staggering and relentlessly mounting. As of April 20/04/ 2020, there have been more than 2314621 individuals in more than 210 countries with confirmed COVID-19, of whom more than 157847 have died [2]. While it predominantly affects the respiratory system, SARS-CoV-2 has also been shown to affect cardiovascular damage [3]. It appears that preexisting cardiovascular disease (CVD) and CV risk factors enhance vulnerability to COVID-19. Further, COVID-19 can worsen underlying CVD and even precipitate de novo cardiac complications [4]. This review highlights overview of various CV manifestations in patients presenting with COVID-19 including the impact of pre-existing CVD and new onset cardiac complications on clinical outcomes. As our knowledge of COVID-19 is still evolving, the information in subsequent text is based mainly on the limited early experience with COVID-19 and learning from outbreaks of SARS and Middle East respiratory syndrome (MERS).
PATHOPHYSIOLOGY AND CLINICAL FEATURE
SARS-CoV2, like other members of the Coronaviridae family, is an enveloped virus with nonsegmented, single stranded, positive-sense RNA genome [5]. Seven species of these beta-coronaviruses are known to cause human infections, with four mainly causing mild flulike symptoms and the remaining three resulting in potentially fatal illnesses (SARS, MERS and the ongoing COVID-19). Angiotensin- converting enzyme 2 (ACE2) is a membrane bound aminopeptidase that has a vital role in the CV and immune systems [6]. ACE2 is involved in heart function and the development of hypertension and diabetes mellitus. ACE2 has been identified as a functional receptor for coronaviruses as well [6]. SARS-CoV2 binds with ACE2 protein and enters cells via receptor-mediated endocytosis. ACE2 is highly expressed in lung alveolar cells, providing the main entry site for the virus into human hosts [7]. ACE2 also serves a role in lung protection and therefore viral binding to this receptor is followed by deregulation of a lung protective pathway, contributing to viral pathogenicity [8]. The infectivity of COVID-19 is greater than that of influenza, with an estimated R0 value (the basic reproduction number, representing viral infectivity) of 2.28 [9]. Notably, the death rate associated with COVID-19 is also considerably higher compared with that of influenza, and may reach even much higher in elderly patients, those with comorbidities [10]. As many as 20% individuals may have asymptomatic infection significantly contributing to further spread of infection [11].
The clinical presentation for COVID-19 is quite fluctuating. A large study from the Chinese Center for Disease Control and Prevention reported the clinical severity as mild in 81.4%, severe in 13.9% and critical in 4.7% [1]. The clinical characteristics of mild COVID-19 include symptoms common to other viral infections (i.e. fever, cough, dyspnea, myalgias, fatigue, and diarrhea) as well as laboratory abnormalities such as lymphopenia although knowledge of the clinical feature of the disease is unfolding daily [4, 8]. In severe cases, COVID-19 may manifest as pneumonia, the acute respiratory distress syndrome (ARDS), with or without both distributive and cardiogenic shock, to which elderly people with underlying co-morbidities are the most susceptible [4, 5, 12]. The patients with the most severe clinical presentations are at risk for superimposed infections, and carry worse outcomes [12, 13]. Identification of prognostic factors associated with morbidity and mortality are crucial as an extremely large number of patients have been diagnosed with COVID-19. As of today there exist no approved preventative vaccines or therapies for this disease, although several are being actively examined [14].
CARDIOVASCULAR CO-MORBIDITIES
Mechanisms that lead to CVD are increasingly recognized to overlap with pathways that regulate immune function. CVD risk factors such as adults older than 65 years, diabetes and hyperlipidemia, impaired immune function, and conversely, dysregulated immunologic status correspond with elevated risk of CVD [15, 16, 17, 18]. Also, the presence of CVD itself may be a marker of accelerated immunologic aging/dysregulation and relate indirectly to COVID-19 prognosis. Higher expression of ACE2 in patients with hypertension and CVD has been postulated to enhance susceptibility to SARS-CoV2, although the data are conflicting [19]. A meta-analysis of six studies incorporating 1,527 COVID-19 patients reported the prevalence of hypertension, cardiac and cerebrovascular disease, and diabetes to be 17.1%, 16.4%, and 9.7%, respectively [20].
CARDIOVASCULAR CO-MORBIDITIES
Table 1 summarizes some of the potential CV manifestations which may result from COVID-19 infection. Existing reports suggest CV complications or exacerbation of preexisting CVD with SARS-CoV2 infection [1, 13].
Table 1. Cardiovascular manifestation of COVID-19
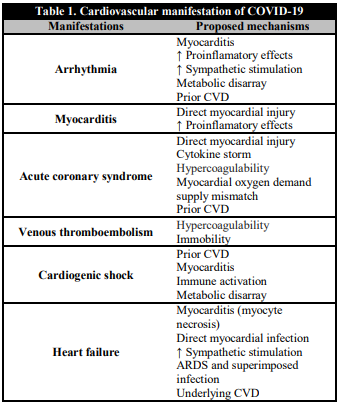
COVID-19: Coronavirus disease 2019; CVD: cardiovascular disease; ARDS: adult respiratory distress syndrome.
ACUTE MYOCARDIAL INJURY
Acute myocardial injury (as defined by an increased troponin level) can occur due to myocardial ischemia or non-ischemic myocardial processes including myocarditis [21, 22, 23, 24]. With severe respiratory infection and hypoxia, especially in the setting of ARDS due to COVID-19, development of myocardial injury is more likely. Elevated serum troponin levels have been described in many patients infected with COVID-19, with significant differences noted between patients who died and those who survived. A meta-analysis incorporating 341 patients, reported higher cardiac troponin I levels in those with severe illness compared to those with non severe disease [25]. Reports have also suggested that acute cardiac injury–which includes not only elevation of cardiac biomarkers to >99th percentile of the upper reference limit, but also electrocardiographic and echocardiographic abnormalities–is highly prevalent in patients with COVID-19 and is associated with more severe disease and worse prognosis. Such injury occurs in 7-17% of hospitalized patients with the disease [4, 5, 23] and is significantly more common in patients admitted to the ICU and among those who died [7, 25].
MYOCARDITIS
Elevated cardiac biomarkers, new-onset cardiac arrhythmias, and acute-onset heart failure symptoms in a SARS-CoV-2 patient should raise clinical suspicion for acute myocarditis. Prior studies with MERS-CoV have demonstrated evidence of acute myocarditis using cardiac magnetic resonance imaging [26], and myocardial inflammation and damage have been reported with COVID-19 infection. Among 68 deaths in a case series of 150 patients with COVID-19, 7% were attributed to myocarditis with circulatory failure and in 33% of cases where myocarditis may have played a contributing role to the patient's death [13]. There are reports of fulminant myocarditis in the setting of high viral load with autopsy findings of inflammatory mononuclear infiltrate in myocardial tissue [27, 28, 29]. Pericardial involvement with tamponade has been described recently [30].
ACUTE CORONARY SYNDROME
Case reports of acute coronary syndromes (ACS) (Type 1 myocardial infarction (MI)) in the setting of COVID-19 have yet to be published. The majority of MIs are type 2 and related to the primary infection, hemodynamic, and respiratory derangement. The profound inflammatory response and hemodynamic changes associated with severe disease may increase risk of plaque rupture in susceptible patients (Table 1) [23]. The patients with acute respiratory infections are at elevated risk for subsequently developing acute myocardial infarction after influenza (and after non-influenza viral illnesses including other coronavirus species as demonstrated by Kwong and colleagues [31]. COVID-19-associated myocarditis needs to be carefully considered as a diagnostic possibility before reperfusion therapy is considered in ST elevation MI (STEMI). A Case report from Italy describes a patient with COVID-19 presenting with ECG findings suggesting non-STEMI without obstructive coronary artery disease [32]. The cases of ACS in the setting of COVID-19 are likely to develop as virus continues to infect patients with significant CV risk factors, or established CVD. Because of logistical challenges associated with limited testing and cardiac catheterization laboratory availability in the setting of this outbreak, the true prevalence may be underreported.
CARDIAC ARRHYTHMIA
Cardiac arrhythmias are another common CV manifestation described in patients with COVID-19 infection. Wang et al, reported cardiac arrhythmia in 16.7% of 138 patients in a Chinese cohort and it was more common in ICU patients compared to non-ICU patients (44.4% vs. 6.9%) [4]. The authors did not specify arrhythmia types or their median duration. Another study demonstrated that cardiac arrhythmias were significantly more common in patients with critical forms of COVID-19 than in mild and moderate cases [34]. Palpitations were part of the presenting symptomatology in 7.3% of patients in a cohort of 137 patients [35]. The metabolic abnormalities, hypoxia, neurohormonal or inflammatory stress could in part, contribute to high prevalence of arrhythmia in the setting of viral infection. Telemetry monitoring for arrhythmias might be reasonable for all COVID-19 patients but especially recommended for those with pre-existing CVD, elevation in cardiac biomarkers, or with severe forms of COVID-19. The new onset of malignant tachyarrhythmias in the setting of troponin elevation should raise suspicion for underlying myocarditis [36].
CARDIOMYOPATHY AND HEART FAILURE
Zhou and colleagues [23] observed heart failure in 23.0% of patients with COVID-19. Notably, heart failure was more commonly seen in acute kidney injury in this cohort and was more common in patients who died compared to those who did survive (51.9% vs. 11.7%) [23]. Heart failure may be attributed to exacerbation of pre-existing left ventricular dysfunction or new cardiomyopathy (either due to myocarditis or stress cardiomyopathy) [37]. Right heart failure and associated pulmonary hypertension should be also considered, in particular in the context of severe parenchymal lung disease and ARDS [38].
CARDIOGENIC AND MIXED SHOCK
The de novo or coexisting cardiogenic pulmonary edema may manifest as ground-glass opacities on chest imaging and hypoxemia akin to ARDS caused by COVID-19. As such, it is important consider cardiogenic or mixed cardiac plus primary pulmonary causes of respiratory manifestations in COVID-19. Cardiac injury may occur directly as the result of viral invasion or indirectly by the cytokine storm induced by COVID-19 [39]. The Berlin criteria utilize timing of symptom onset, imaging with bilateral pulmonary opacities, and lack of volume overload to identify patients with ARDS [40]. Serum brain natriuretic peptide (BNP) and echocardiography can help clarify the diagnosis in many cases [41, 42]. However, if these tests are unclear and there remains concern for mixed presentation, pulmonary artery catheterization may be required in select cases to assess filling pressures, cardiac output, and to guide decision-making, given the different management approaches for ARDS and cardiogenic shock. Finally, it is crucial to determine whether or not a concomitant cardiogenic component is present when considering mechanical respiratory and circulatory support with extracorporeal membranous oxygenation (ECMO) or other techniques, as this may lead to changes in device selection (e.g. venovenous vs. venoarterial ECMO) [38]. The prognosis may be guarded regardless of ECMO support in the most severe of infections with ARDS. In a case series 83.3% of critical patients treated with ECMO did not survive. Further studies are warranted regarding the utility of ECMO in advanced COVID-19, including which patients may (or may not) benefit and whether concomitant left ventricular venting should be done [43].
VENOUS THROMOEMBOLISM
Among severely ill patients with COVID-19, a variety of potential risk factors for venous thromboembolism (VTE) exist, including infection, immobilization, respiratory failure, mechanical ventilation, and central venous catheter use [4, 43]. There are reports of abnormal coagulation parameters in hospitalized patients with severe disease [44, 45]. In a multicenter retrospective cohort study from China, elevated D-dimer levels (> 1g/L) were strongly associated with in-hospital death [23]. In another study comparing COVID-19 survivors to non-survivors, non-survivors had significantly higher D-dimer and fibrin degradation products (FDP) levels and 71.4% of non-survivors met clinical criteria for disseminated intravascular coagulation (DIC) during the course of their disease [44]. In addition to DIC, critically ill patients with prolonged immobilization are inherently at high risk for VTE. Vascular inflammation may also contribute to the hypercoagulable state and endothelial dysfunction in such patients. The assessment of VTE and bleeding risks regularly is essential especially in severely and critically ill patients. Additionally, it was found that patients with COVID-19 with a high risk of VTE had poorer outcomes than patients with a low risk, suggesting that these patients might require increased attention in case of rapid deterioration [46]. VTE should be considered in critically ill COVID-19 patients demonstrating clinical deterioration with hypoxia or hemodynamic instability. The contemporary guideline endorsed strategies should be considered till optimal prophylactic regimen is known for such patients [47]. Given the drug-drug interactions between some antiviral treatments and direct oral anticoagulants, low molecular weight heparins, or unfractionated heparin with or without mechanical prophylaxis are likely to be preferred in critically ill patients [38].
HEART TRANSPLANTATION IN THE ERA OF COVID-19
One area of cardiovascular medicine that remains especially vulnerable in COVID pandemics is that of heart transplantation. The individuals waiting or post heart transplantation both are at risk. The eminent risk for the later is more obvious owing to their immunocompromised state. Two heart transplant recipients from the Hubei province of China, one with mild and one with severe disease, presented with fever and had laboratory and CT scans that were similar to non-immunosuppressed individuals with bilateral ground glass opacities in a peripheral distribution. Both were managed by withholding baseline immunosuppressive regimens and treating with high dose steroids, intravenous immunoglobulin, and antibiotics, and both survived without evidence of allograft rejection [48, 49]. A recently published survey of 87 heart transplant recipients in China, did not find a higher risk of infection with SARS-CoV-2 if routine preventive measures were undertaken, and while encouraging, this needs to be confirmed in larger populations [50]. The safety of patients awaiting heart transplantation has also been jeopardized by the COVID-19 pandemic due to potential transmission from the donor to the recipient. Very little is known about this novel virus to reliably estimate the risk of transmission through donor organs. Currently it is recommended to continue heart transplantation without changes in immunosuppression provided the recipient has tested positive for SARS-CoV-2 and has not had exposure to or symptoms of COVID-19 in the prior two to four weeks [51, 52]. Major societal recommendations include avoiding donors with known or suspected COVID-19 and if a donor had COVID-19, they should be COVID-19 free (by PCR) for at least 14 days [53, 54]. Recommended management of transplant recipients developing COVID-19 is primarily supportive care and continuation of immunosuppression for mild COVID-19 with reduction of the anti-metabolite (mycophenolate or azathioprine) and further treatment based on disease severity [39].
DRUG THERAPY AND CARDIAC CONCERN
Preventive measures are the best strategies for COVID-19 at this time as there no specific effective therapies. While vaccines and monoclonal antibodies against SARS-CoV-2 are in development, a number of other investigational therapies targeting SARS-CoV-2 cell invasion and replication may be considered. As these drugs are being studied, it is important to review the potential CV side effects and interactions with other CV medications.
ANTIVIRAL THERAPY
Ribavirin and lopinavir/ritonavir are the antiviral drugs under investigation in clinical trials for COVID-19 and have been used for years in the treatment for hepatitis C and HIV, respectively [55, 56]. Ribavirin lacks characterized direct CV toxicity. Lopinavir/ritonavir may result in QT and PR interval prolongation, especially in patients who have a baseline abnormality (long QT) or those who are at risk for conduction abnormalities.56 Both ribavirin and lopinavir/ritonavir have the potential to affect anticoagulant dosing: ribavirin has variable effects on warfarin dosing [57] and lopinavir/ritonavir may require dose reductions or avoidance of CYP3A mediated drugs such as rivaroxaban and apixaban [58, 59].
Lopinavir/ritonavir reduces serum concentrations of the active metabolites of clopidogrel and prasugrel and reduces serum concentrations of ticagrelor by influencing the activity of P2Y12 inhibitors through CYP3A4 inhibition given the increase in serum ticagrelor levels with such medications [60, 61]. concomitant use of ticagrelor should be avoided due to excess in bleeding risk. There is evidence that clopidogrel may not always provide sufficient platelet inhibition in the setting of concomitant administration of lopinavir/ritonavir. Prasugrel can be used in such scenario; however, if contraindicated (i.e. history of stroke or TIA, low body mass index, or active pathological bleeding), a testing-guided approach (e.g. with P2Y12 platelet function assays) may be considered with alternate antiplatelet agents [62, 63]. Finally cangrelor, is independent of hepatic function, therefore a drug interaction is not expected.
Statins can result in myopathy when administered together with lopinavir/ritonavir. Lovastatin and simvastatin, in particular, are contraindicated for co-administration with lopinavir/ritonavir due to risk of rhabdomyolysis. The dose of other statins, including atorvastatin and rosuvastatin, should be the lowest possible while on lopinavir/ritonavir therapy [56].
ANTI-MALARIAL DRUGS
Chloroquine, a widely used anti-malarial drug, is known to block virus infection by increasing the endosomal pH required for virus/cell fusion and interfering with the glycosylation of cellular receptors of SARS-CoV [64-66]. Chloroquine and the closely related hydroxychloroquine have the potential for myocardial toxicity. Risk factors include long-term exposure (>3 months), higher dose, underlying cardiac disease, and renal insufficiency [67]. Chloroquine cardiac toxicity presents as restrictive or dilated cardiomyopathy or conduction abnormalities thought to be due to intracellular inhibition of lysosomal enzymes in the myocyte [67, 68]. In addition, due to effects of chloroquine can increase serum level of, beta-blockers metabolized via CYP2D6 (such as metoprolol, carvedilol, propranolol, or labetalol) warranting careful monitoring for heart rate and blood pressure shifts. Lastly, both agents are associated with increased risk of torsade des pointes in patients with electrolyte abnormalities or with concomitant use of QT prolonging agents.
ANGIOTENSIN-CONVERTING ENZYME INHIBITORS AND ANGIOTENSIN RECEPTOR BLOCKERS
There has been a tremendous contemplation surrounding the potential adverse effects of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) in COVID-19 patients. As the ACE2 receptor is the mechanism of entry for SARS-CoV2, some data suggest that ACEIs and ARBs may upregulate ACE2, thereby increasing susceptibility to the virus. In contrast, other studies reveal that ACEIs and ARBs may be protective against viral pneumonias [69, 70, 71]. Similarly, there have been studies in animals infected with SARS-CoV-2 where these medications have been shown to potentiate lung protective action, but to date human data are lacking [72]. Hence, the authors recommend that physicians and patients continue their treatments with ACEIs and ARBs because there is insufficient data to suggest any mechanistic connections between ACEIs/ARBs therapy with contracting COVID-19 or with severity illness once infected.
STEROIDS
Methylprednisolone, used in treatment of severe cases of COVID-19 that are complicated by ARDS [28] is known to cause fluid retention, electrolyte imbalance, and hypertension as direct CV effects, and also may interact with warfarin via an undescribed mechanism.
COVID-19 AND MANAGEMENT IMPLICATIONS
The overall management principles for patients presenting with COVID-19 who develop CV complications or who have pre-existing CVD are same as for any other patient without COVID-19.
CONSIDERATIONS FOR HEALTHCARE WORKERS
All healthcare personnel engaged in the care of COVID-19 patients must observe necessary precautions at all times. All of them should be trained in donning, usage, and doffing of the personal protective equipment (PPE). Since the very beginning of the COVID-19 outbreak, the WHO maintains the recommendation of using medical masks for regular care of COVID-19 patients in the context of droplet and contact precautions, and respirators (N95, FFP2 or FFP3) for circumstances and settings where aerosol generation can occur such as transesophageal echocardiography, endotracheal intubation, cardiopulmonary resuscitation and bag mask ventilation [73]. PPE with aerosolization protection for the entire catheterization laboratory staff during PCI for all STEMI patients during this COVID-19 pandemic is recommended as per the previously published ACC/SCAI guidelines for managing CCL patients during the COVID-19 epidemic [74]. It is essential to consider minimization of the number of healthcare staff potentially exposed during invasive cardiovascular procedures; this may include limiting the involvement of trainees in high risk procedures and patients. Healthcare workers in the setting of cardiac arrest and chest compressions should be encouraged to use external mechanical compression devices to minimize direct contact with infected patients (Table 2). Elective procedures should be cancelled or kept minimum during this pandemic considering limited availability of catheterization laboratory because of the necessary downtime required for cleaning. There have been reports of centers in China converting positive pressure ventilation configured operating theatres to negative pressure isolation in the setting of COVID-19 [75].
Table 2. Protection of healthcare workers
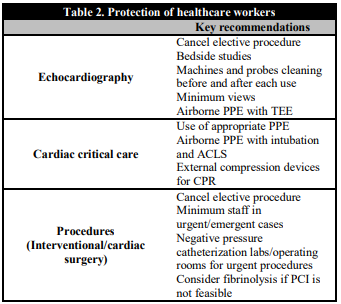
ACLS: Advanced cardiac life support; CPR: Cardiopulmonary resuscitation; PCI: Percutaneous coronary intervention; PPE: Personal protective equipment; TEE: transesophageal echocardiography.
Telemedicine is ideal in public health crises as it allows for patients to be triaged while minimizing exposure of patients and health care workers to potential infection [76]. Other essential principles are to respect social distancing and limit elective cardiac catheterization, operating room and echocardiographic procedures. For this, number of required personal should be kept to a minimum (Table 2).
CONSIDERATIONS FOR MANAGEMENT OF NON-INFECTED CV PATIENTS
Given the clear implications of this pandemic on CV care, numerous societies have already provided guidance statements, which are summarized in Table 3. The ACC Clinical Bulletin discusses about key implications and recommendations for CV care of COVID-19 patients [77]. The ESC Council on Hypertension and European Society of Hypertension statement sheds light on ACEI and ARB therapy in such pandemic [78]. The various societies are of the views that further data would be vital for adjusting regimens of these agents during the current outbreak [78, 79, 80, 81]. The hospitals may have to prioritize the treatment of severe and high-risk patients as the pandemic surges. While preserving limited in-patient resources and minimizing provider and patient exposures, specific protocols will be required to be developed. There are reports of individual centers utilizing fibrinolytic therapy for STEMI if delays to primary PCI are anticipated when hospitals are at capacity or staffing for the catheterization lab is inadequate [83]. Primary PCI remains the standard of care for STEMI patients at PCI capable hospitals when it can be provided in a timely fashion, with an expert team outfitted with PPE in a dedicated cardiac catheterization room. Fibrinolytic therapy may be adopted at non-PCI capable referral hospitals or in specific situations where primary PCI cannot be executed or is not deemed feasible. NSTEMI patients who are COVID-19 positive or probable are optimally managed with medical therapy in the absence of hemodynamic instability or ongoing ischemia. Finally, the care of patients will require the integrated approach incorporating expertise of many specialty services including pulmonology, critical care, infectious diseases, cardiology, surgery, pharmacy, and hospital administration among others [38].
Table 3. Guideline considerations with regard to CVD and COVID-19

COVID-19: Coronavirus disease 2019; ACC: American College of Cardiology; ACEP: American College of Emergency Physicians; SCAI: Society for Cardiovascular Angiography and Interventions; CV: Cardiovascular; CVD: Cardiovascular disease; PCI: Percutaneous coronary intervention; CABG: Coronary artery by-pass grafting; ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin receptor blocker; ARNI: Angiotensin receptor neprylisin inhibitor; ESC: European Society of Cardiology; STEMI: ST elevation myocardial infarction; NSTEMI: Non-ST elevation myocardial infarction.
CONCLUSIONS
Our knowledge of COVID19 pandemic and its CV implications is evolving by the hour. CV co-morbidities are common in patients with COVID-19 and such patients pose higher risk of morbidity and mortality. However, it is yet to be known if the presence of CV comorbid conditions poses independent risk or whether this is mediated by other factors. The CV community will play a key role in the management and treatment of patients contracted with this disease, and in addition in providing continuity of care to none infected patients with underlying CVD. Furthermore, it is important for the clinicians to stress the urgent need for high quality research into the interplay between COVID-19 and CVD and the efficacy and safety of new therapies, which will be crucial to the treatment of infected patients.














