INTRODUCTION
SARS-COV-2 is the causative agent of COVID-19 and the seventh form of coronavirus which took its origin in Wuhan, Hubei province of China in December 2019. The disease has been declared as a pandemic on 11th March 2020 by WHO, and as on 24th August 2020, there were 23,616,858 positive cases, 813,068 deaths and 16,105,572 recoveries worldwide [1, 2]. Clinical symptoms of COVID-19 include fever, cough, tiredness, and shortness of breath, sore throat, running nose and headache while the risk of morbidity and mortality include age, sex, and presence of co-morbidities such as cardiovascular diseases, diabetes, respiratory diseases, cancer, liver and kidney diseases [3, 4].
According to the International Society of Blood Transfusion, more than 30 blood groups exist and include the ABO and Rh blood groups [5]. The ABO and Rh blood group was discovered by Landsteiner in 1901 and 1941 respectively [6] and consists of the A, B, and H carbohydrate antigens and antibodies against these antigens [7]. The A, B and D blood antigens are found on the surface of the red blood cells and their presence or absence determines the ABO and Rh blood grouping of an individual [8]. The blood group antigens are glycoproteins and glycolipids and are genetically controlled and inherited in varying frequencies across human populations [8, 9]. Human ABO blood is located on chromosome 9 (9q34.2) [10] and are of four types: A, B, AB and O.
Though studies are currently ongoing to identify biological markers that can predict individual's susceptibility to SARS-COV-2, severity and COVID-19 clinical outcome have been associated with serum levels of some laboratory parameters [11, 12, 13]. Liver function tests have been used to assess the association between liver injury and COVID 19 [14].
The ABO blood group has been linked to diseases such as Norovirus [15], Influenza [16], Plasmodium falciparum infection and cancer [17]. Now there are growing evidences that showed the susceptibility to SARS-COV-2 is related to the ABO blood group of an individual [18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33]. Also, conflicting evidences exist on the relationship between ABO blood group and the severity and clinical outcome of COVID-19 disease. The aim of the current study was to carry out a systematic review and meta-analysis of all the studies that investigated the relationship between ABO blood group and COVID-19 for better understanding and guide.
MATERIALS AND METHODS
An electronic literature search was conducted by two independent reviewers using the online databases of Pubmed/Medline, Google Scholar, Journal Storage (JSTOR) and African Journal OnLine (AJOL) from 2nd August, 2020 for relevant publications published up to 25th August, 2020. “ABO blood group” during the search protocol was combined with either “COVID-19”, “SARS-COV-2” or “coronavirus” to identify studies published online. Eligible studies were further snowballed to identify additional articles.
Participants in this study were individuals with known ABO blood type who were documented to have SARS-COV-2 infection; whereas controls are individuals with known ABO blood group who were not SARS-COV-2 positive.
From this definition, studies were included if they:
Examined the association between ABO blood groups and either SARS-COV-2 infection or/and COVID-19 severity or/and death due to COVID-19,
Provided original data 3. Provided information on normal individuals with their ABO blood type known,
Were case-control, case series, cohort and cross-sectional in design (reports, correspondence and letters to the editors were also included if reporting original data).
Our exclusion criteria include:
Articles irrelevant to the subjects of the study (animal models, studies on COVID-19 that were not associated with ABO blood group and vice versa, studies on other strains of coronaviruses other than COVID-19),
Studies whose data are not extractable,
Duplicated studies (where we included articles once even when they were found in different databases),
Reviews and Figures,
Studies not retrievable in English language.
There were no restrictions on year, age, sex, gender, comorbidities, SARS-COV-2/blood type testing methods, testing time, sample size, publication status, race, country/region and follow-up time.
Eligible articles using the inclusion and exclusion criteria were obtained by two independent reviewers through screening of the titles and abstracts whereas detailed information was obtained through careful review of the full-text. Any discrepancy was resolved through consensus. The data extraction format used in this study was similar to that reported by Wu et al. [18]. The following information were extracted: the first author and year of publication, country of participants, sample size and sample design, gender ratio, live controls and dead cases, non-severe controls and severe cases, asymptomatic controls and symptomatic cases. All the extracted data were transferred into an Excel spreadsheet. Any controversy to include or exclude any study was resolved through consensus and when it cannot be achieved, the main reviewer made the final decision. The data screening, selection and extraction processes were manually done and the entire process was done without blinding. The primary outcome measures were to investigate the association between ABO blood group and: 1. COVID-19 susceptibility; 2. COVID-19 severity; and 3. Mortality due to COVID-19. COVID-19 severity is defined as hospitalization with respiratory failure with subjects admitted in the intensive care unit of the hospitals. Figure 1 contains the flow chart of the study selection process.
In consultation with the other authors, the main reviewer carried out the quality appraisal of included studies using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies recommended by National Institute for Health (NIH). The quality tool is comprised of 14 items/questions with a total score of 14. Each study was scored on a Yes (1) or No (0), and others [not applicable (NA), not reported (NR) or cannot determine (CD)]. Not applicable was used where some elements of the criteria did not apply as seen in Table 1.
Table 1. Quality Assesment Tool for Observational Cohort and Cross-Sectional Studies
Other includes not applicable, not reported and undetermined data.
STATISTICAL ANALYSIS
Data analysis for included studies was performed using Review Manager Software (version 5.3.5). The random-effect model was used to obtain the pooled odds ratios (ORs) and 95% confidence interval (CIs) and was used to measure the association between SARS-COV-2 infection, COVID-19 severity, COVID-19 related deaths and ABO blood group. Increased or decreased risk of COVID-19 infection, severity or death was indicated when the OR was greater or less than 1 respectively. Test of heterogeneity between studies was assessed using the chi-squared test and I-squared (I2) statistic. Results of the meta-analysis were presented graphically using forest plots. In the forest plots, the weight of each study and the 95% CIs determined the size of the squares and the lines beside it respectively. The level of statistical significance was set at P < 0.05.
RESULTS
A total of 585 articles were initially identified after search of the databases and were reduced to 509 articles following duplicates removal as shown in Figure 1. After a thorough scan of the abstracts using the study inclusion criteria another set of 467 articles were removed. The full texts of the remaining forty-two articles were critically evaluated resulting in twenty-eight articles being excluded using the study exclusion criteria. One study that met inclusion criteria for qualitative synthesis was later dropped for meta-analysis due to low quality (quality score of 3) as seen in Table 1 and data on ABO blood group and COVID-19 were not clearly extractable [19]. Finally, a total of 14 [20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33] studies were included in this systematic review and meta-analysis.
CHARACTERISTICS OF INCLUDED STUDIES FOR META-ANALYSIS
The basic characteristics of included studies are shown in Table 2. Fourteen eligible studies including 13189 SARS-COV-2 positive cases and 60745 controls were included in our meta-analysis. The studies were all published in year 2020 (Table 2). On study location, six of the studies were carried out in China [21, 23, 28, 30, 31, 33], four in US [24, 25, 26, 32], one each in France [22], Turkey [29] and Iran [20]. One of the studies was multi-country in nature (involving Spain and Italy) [27]. There were 13 retrospective studies and one cross-sectional study. The NIH quality assessment score of selected studies ranged from 7 to 10 points (the highest possible score is 14), with mean and median of 8 each (Table 2). Of all studies, 12 were original articles while one each was a correspondence [26] and a letter to an editor [25]. The case groups were COVID-19 infected individuals admitted in the hospital. Meanwhile, for the control subjects, three studies used data from blood donor populations/databases [20, 27, 29], three studies used data from the general population [30, 32, 33] while in one [22], and in four other studies [23, 24, 25, 28] control data were obtained from populations with same level of exposure and hospitalized patients who were in admission before COVID-19 episode, respectively. Control data was obtained from asy,ptomatic COVID-19 carriers in one study [21] while two studies [26, 31] did not use control.
Table 2. Characteristics of included studies and subgroups
M: Male; F: Female; NR: Not reported. Zhao et al. [33] in one (1) manuscript presented three (3) studies in 3 different areas in China and presented differently here. Also, all control participants are COVID-19 negative subjects apart from those reported by Zhou et al [21] which are asymptomatic carriers.
From Table 3, twelve evidences reported data on the association between ABO blood group and SARS-COV-2 susceptibility. As shown in Figure 2, the risk of contracting SARS-COV-2 infection was found to be significantly higher among individuals of blood group A (OR: 1.24, 95%Cl: 1.09 - 1.41, P = 0.001). The heterogeneity among the studies was found to be significantly high (I-squared = 83%, P ˂ 0.00001) using the random-effect model. However, the risk of SARS-COV-2 infection was found to be significantly decreased among blood group O individuals (OR: 0.78, 95% Cl: 0.68 - 0.89, P = 0.0003); a significant heterogeneity (I-squared = 84%, P ˂ 0.00001) was also observed between studies using the random-effect model. When compared with other ABO blood groups and applying random-effects models (for B: I-squared = 35%, P = 0.10; for AB: I- squared = 70%, P ˂ 0.0001 ), the odds of SARS-COV-2 infection seemed to be increased among individuals with blood group B (OR: 1.05, 95%Cl: 0.97 – 1.14, P = 0.25) and blood group AB (OR: 1.12, 95%Cl: 0.92 - 1.36, P = 0.27). However, the findings were of no statistical significance. Table 4 contains the result of the subgroup analysis. The observed association between blood group A and SARS-COV-2 infection remained stable in studies carried out in China, Europe, and across all sample sizes. Meanwhile, the inverse relationship between blood group O and SARS-COV-2 infection was unchanged among studies carried out in Europe, Iran, China and studies with sample sizes ˃ 2000 (Table 4). However, increased risk of SARS-COV-2 infection in blood group B subjects was found to be statistically significant among studies carried out in USA (OR: 1.13, 95CI: 1.03 - 1.23) and those with sample sizes > 2000 (OR: 1.09, 95CI: 1.02 - 1.16) but remained unchnaged and non-significant in studies done in China, Europe, Iran and those with sample sizes < 2000 (Table 4). Furthermore, significant risk of SARS-COV-2 infection in blood group AB subjects was only found among Iranians (OR: 1.95, 95CI: 1.15 - 3.30) (Table 4). In sensitivity analysis is shown in Table 7 using Sensitivity analysis (leave-one-out approach), the high heterogeneity observed in blood groups A, AB and O (Figure 2) could not be accounted for by the individual studies as the I2 and the pooled estimate remained unchanged. Besides, low heterogeneity was observed among studies carried out in USA while remaining high among studies done in China and Europe with the exception of blood group A and AB respectively (Table 4).
Table 3. Studies showing results for COVID-19 susceptibility and blood group distribution
Leaf et al. [26] and Zeng et al. [31] were excluded because there are no control data provided for comparison and Zhao et al., in one (1) manuscript presented three (3) studies in 3 different areas referenced as [33], {33} and │33│.
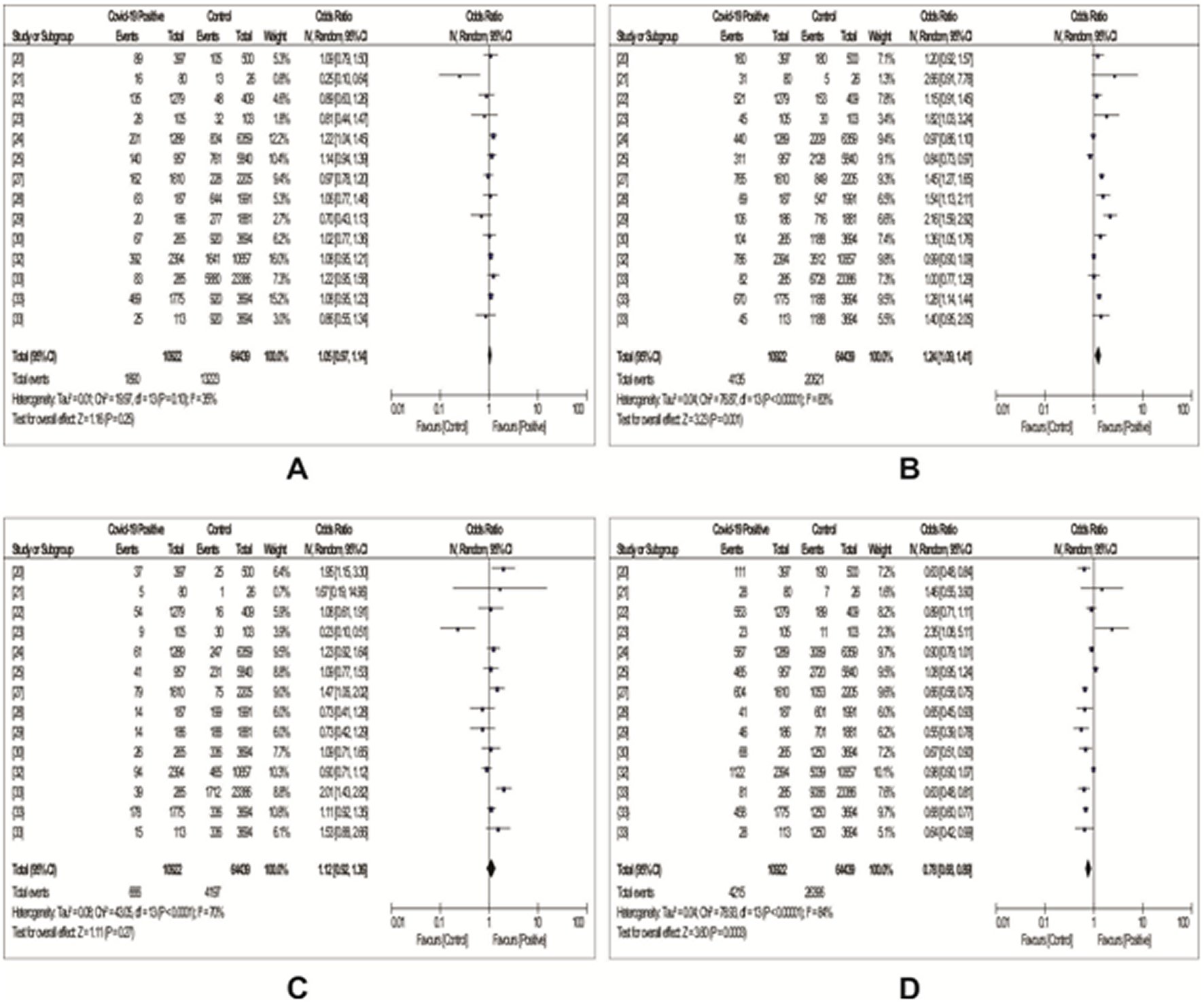
Figure 2. Forest plots with odd ratios showing the association between ABO blood groups and COVID-19 susceptibility.A: A blood group; B: B blood group; C: AB blood group; D: O blood group.
Table 7. Results from sensitivity analysis leav-one-out-method: pooled/OR and 95% confidence interval calculated omitting each study in sturn
Leaf et al. [26] and Zeng et al. [31] were not included in the subgroup analysis because they were not part of studies included for susceptibility testing (Table 2).
A total of nine studies reported data on the association between COVID-19 severity and ABO blood group, as were shown in Table 5. Using the random-effects model, we observed from our meta-analysis as shown in Figure 3 an increased risk of COVID-19 severity among individuals of blood group B (OR: 1.15, 95%Cl: 0.90 - 1.45, P = 0.26), those of blood group AB (OR: 1.12, 95%Cl: 0.78 - 1.60, P = 0.55) and blood group O (OR: 1.03, 95%Cl: 0.88 - 1.21, P = 0.73). Observed also was a decreased risk of COVID-19 severity among individuals of blood group A (OR: 0.96, 95%Cl: 0.74 - 1.26, P = 0.78). However, none of the observed results were of statistical significance. Meanwhile, heterogeneity between the studies were generally low (Figure 3).
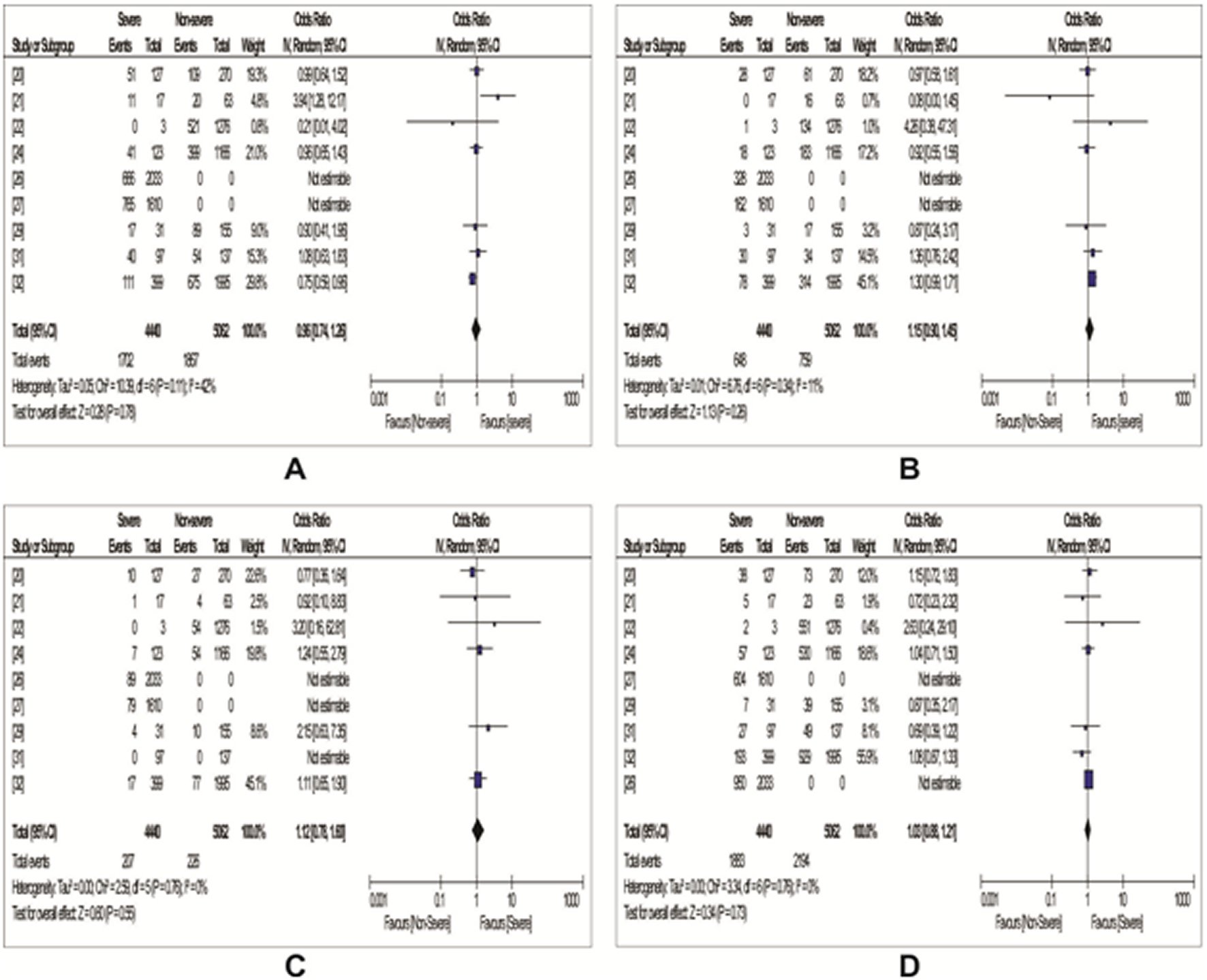
Figure 3. Forest plots with odd ratios showing the association between ABO blood groups and COVID-19 severity.A: A blood group; B: B blood group; C: AB blood group; D: O blood group.
Eight studies reported data on the association between COVID-19 mortality and ABO blood group, out of which, complete data on blood group AB was seen in seven of the studies as were shown in Table 6. From Figure 4 using random-effects model, our meta-analysis showed a non-statistically significant increased risk of death among people with A blood group (OR: 1.26, 95%Cl: 0.93 – 1.70, P=0.13) and blood group AB (OR: 1.23, 95%Cl: 0.93 – 1.62, P=0.15). However, individuals with O blood group and blood group B were found to have a non-statistically significant decreased risk of death [blood group O: OR: 0.93 (95%Cl: 0.75 – 1.17) , P=0.55; blood group B : OR: 0.77, 95%Cl: 0.49 – 1.21, P=0.25) (Figure 4).
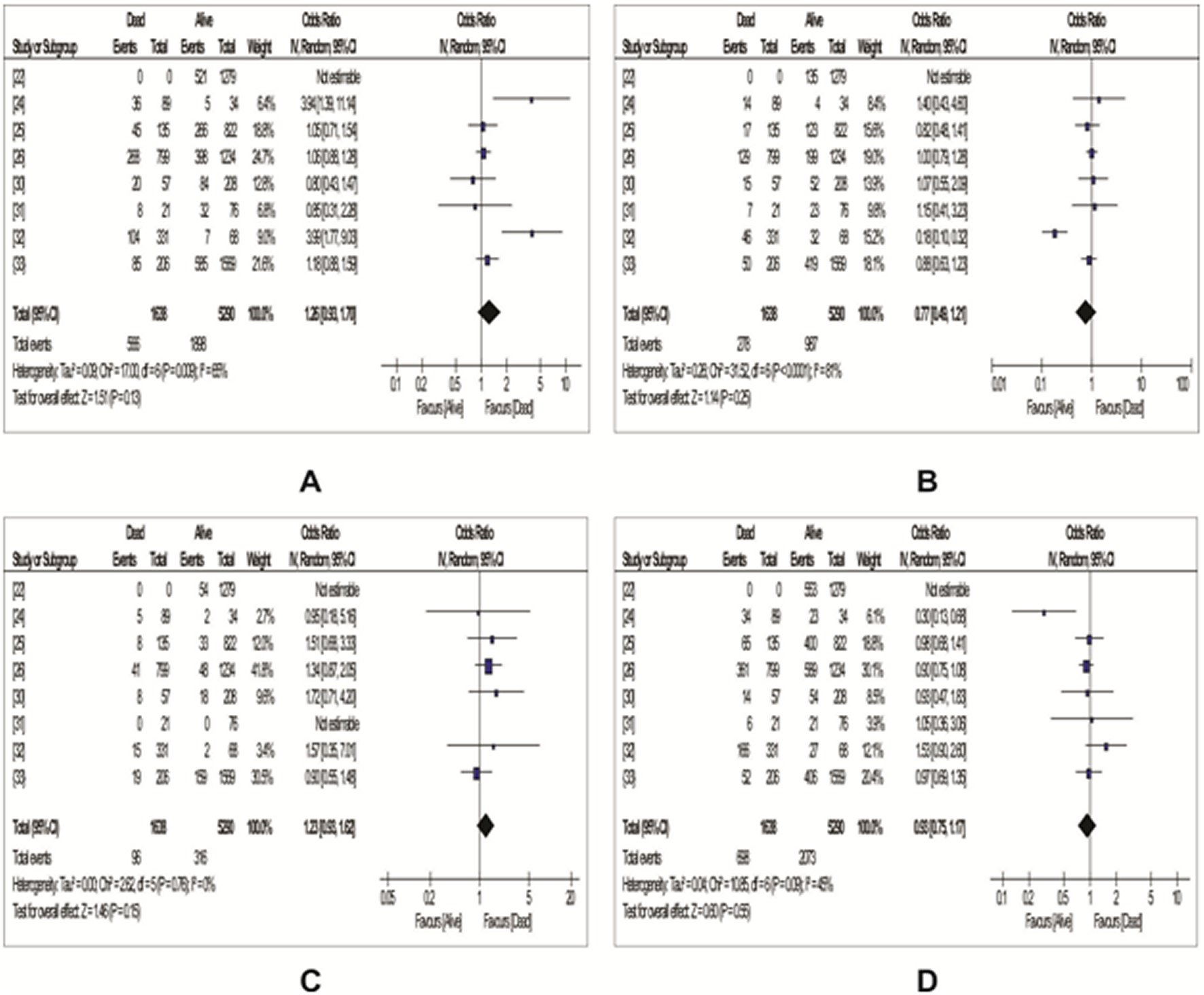
Figure 4. Forest plots with odd ratios showing the association between ABO blood groups and COVID-19 mortality.A: A blood group; B: B blood group; C: AB blood group; D: O blood group.
DISCUSSION
From the analysis of 14 observational studies, we concluded the existence of potential relationship between COVID-19 susceptibility and ABO blood group of individuals. Also, from the study we found that individuals with blood group A were at more risk of being infected with SARS-COV-2 than those of non–A blood group whereas blood group O individuals were seen to have a decreased risk of infection than non-O blood type. The findings of this meta-analysis are similar to an earlier review and meta-analysis study by Pourali and colleagues [34] and a recent work done by Wu and colleagues [18]. In another meta-analysis, the risk of SARS-COV-2 infection was also reported to be increased among A blood type individuals (OR: 1.23, 95%CI: 1.09 – 1.40) and decreased in those with O blood type (OR: 0.77, 95% Cl: 0.67-0.88) [35]. The finding of increased odds of SARS-COV-2 infection among A blood group individuals in this study means that such individuals might need stringent measures like strengthened personal protection and vigilant surveillance to reduce the chance of infection.
The finding of no significant association between ABO blood group and COVID-19 severity in this study (Figure 3) which is similar to that reported in a previous review [18]. However, considering individual studies, significant increased risk of severity among blood group A was seen in Zhou et al. [21].
On the association between death due to COVID-19 and ABO blood group, we did not find any significant relationship in all the ABO blood groups (Figure 4). Our finding corroborates that reported in two previous studies [18, 35]. However, in Latz et al., studies [24], the odds of death were significantly high with A blood group and low with O blood group.
The association of ABO blood groups and susceptibility to several infectious diseases has been a matter of debate for decades now. ABO blood group had been associated with increased risk of hepatitis B infection, pancreatic cancer and other cancers [36, 37]. A blood type was associated with pancreatic cancer [OR (95% CI): 1.425 (1.071–1.894)] and also was found to significantly modify the risk of pancreatic cancer among subjects with anti‐hepatitis B core antibody (anti‐HBc) (OR = 1.882, 95% CI, 1.284–2.760) [37]. In another study, the risk of hepatitis B infection was reported to be decreased and increased among B blood type and blood group O individuals respectively [38]. Meanwhile, other studies reported no correlation between HBV, HIV infection and ABO blood group and even identified risk of infection to be higher in blood group O donors than those of AB group [39, 40].
A review study identified studies that postulated correlations between ABO blood group and different infections like Norovirus, Rotavirus, HIV, Influenza virus, severe acute respiratory syndrome coronavirus (SARS-COV) and malaria [41]. According to these studies, the risk of infection was reported to be increased with blood groups O, A, and B. Additionally, the study trying to explain the association between SARS and ABO type reported that among hospital workers who contracted SARS after exposure to a single index patient, resistance to infection were seen among group O individuals whereas about 23/34 of infected individuals were non-O group (groups A, B and AB) [41]. ABO blood group has also been related to the transmission of SARS following the outbreak in Honk Kong [42].
It is still unclear the exact mechanism that explains the association of ABO blood groups with viral infection [37]. The clinical significance of blood group antigens beyond compatible blood transfusion could be due to their being expressed on many tissues in addition to their presence on the surface of human red blood cells [31, 43]. A recent genomic study identified a 3p21.31 gene cluster as a genetic susceptibility locus in patients with Covid-19 with respiratory failure and reported a potential involvement of the ABO blood-group system [27]. ABO antibody titer, secretor status, and incidence of blood group O in the population have also been identified to be some factors that influence the protective role of blood group O to SARS-COV infection [41]. The possibility of natural antibodies to protect against certain viral infection could be associated with the ability of anti-A and anti-B natural antibodies which are found in individuals of blood group O to recognize A and B antigens on virus glycoproteins [44]. Guillon and colleagues explained the ability of anti-A antibodies either in its monoclonal or natural forms blocked the interaction between the angiotensin converting enzyme 2 (ACE2) receptor and the SARS (S) spike protein [44]. Besides, SARS-COV has been shown to bind to ABO carbohydrates and enter cells through the ACE2 receptors found in virtually all cells of major organs in the body [45]. The absence of anti-A and anti-B natural antibodies in O blood type carriers was reported to limit the SARS-COV binding with ABO carbohydrates and ACE2 [34]. In addition to low ACE2 among the O blood type carriers, they have also been found to have a higher interleukin 6 (IL-6) levels than non-type O carriers [45]. Meanwhile, cytokine storm has been associated with severe COVID-19 disease and have been shown to worsen the condition leading to high mortality associated with the disease [46, 47]. Interleukin-6 plays an important role in moderating the inflammation processes, thus, the high level of IL-6 in O blood type individuals could explain their lesser chances of developing severe COVID-19 disease and even death.
The high levels of Willebrand factor and factor III found in subjects of blood group A, B, or AB have also been reported to increase the susceptibility of these individuals to arterial and venous thromboembolism when compared to those of O blood group [37, 48]. Besides, the risk of developing cardiovascular diseases and aggregate disease situations have been linked to the A allele of the ABO blood group [49]. Meanwhile, the identified mechanism of ABO blood group action on infection needs further investigations and confirmations.
The limitations of the study include:
The publication bias was not determined,
We also did not account for other blood groups such as Rhesus and Duffy groups,
There was also possibility of missing out some studies that should have been included despite thorough literature search,
Despite our robust search strategy no study was identified in Africa.
Meanwhile, the strength of our study is derived from being most recent, employed a larger sample size with more regional coverage compared to other published review reports. None of the included studies were of low quality. However, more large scale confirmatory multi-country studies are needed.
CONCLUSIONS
Blood group A is associated with a higher risk of SARS-COV-2 infection whereas risk of infection was lower in blood group O subjects. No statistically significant association was found between ABO blood groups and COVID-19 severity and mortality. The precise role of ABO blood group in COVID-19 susceptibility, severity and mortality requires further research for clarification.