1. INTRODUCTION
Periodontitis is a multifactorial infectious disease associated with bacterial dysbiosis that leads to the loss of tooth-supporting tissues. This destruction of periodontal tissues is mediated by the host's inflammatory response through the release of proinflammatory cytokines and inflammatory mediators in response to bacterial infection. Periodontitis is an important global public health problem due to its high prevalence and because it is the main cause of tooth loss in subjects over 40 years [1].
Vitamin D is a steroid hormone synthesized primarily from human skin and yeast by exposure to sunlight. Other sources of vitamin D are certain foods and nutritional supplements. Vitamin D comprises two fat-soluble compounds: vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). The major biologically active metabolite of vitamin D in plasma is 1,25-dihydroxyvitamin D [1,25(OH). D] and represents the body's measure of vitamin D storage. The most widely used diagnostic marker for vitamin D status determination is the serum circulating 25-hydroxyvitamin D [25(OH)D] levels. Vitamin D deficiencies are generally due to lack of sun exposure or poor nutrition and are higher in females and the elderly [2].
There is no international consensus on optimal, adequate, inadequate, and deficient vitamin D levels. According to the criteria of the Endocrine Society [3], vitamin D deficiency is classified as severe deficiency (<10 ng/mL), deficiency (10-20 ng/mL), insufficiency (21-29 ng/mL), and normal levels (≥30 ng/mL).
Vitamin D plays an important role in several physiological processes such as bone and calcium metabolism, diverse immune functions, and cell growth and differentiation. Specifically, its main function is maintaining serum concentrations of calcium and phosphorus within normal ranges [4].
Vitamin D is also involved in specific immune responses by suppressing T cell proliferation, immunoglobulin secretion, transformation of B cells into plasma cells, and decreased secretion of several pro-inflammatory interleukins (IL-1, IL-6, IL-8, IL-12) or other cytokines such as TNF-alpha. Therefore, adequate vitamin D levels may decrease alveolar bone resorption, increase bone density or negatively influence the course and progression of periodontitis [5]. Low vitamin D levels have been associated with greater periodontal destruction and more severe stages of periodontitis [6]. In contrast, patients with higher vitamin D levels show less bleeding on probing (BoP), lower concentrations of the periodontal pathogen Porphyromonas gingivalis, and a decrease in the inflammatory response of the periodontal tissues [7].
This study aimed to analyze the influence of vitamin D and calcium levels on periodontitis.
2. MATERIAL AND METHODS
All research steps (search, study selection, data extraction, and evaluation) were achieved independently by both authors (ARA and NMEF). Subsequently, together they selected the articles to include in this study.
2.1. SEARCH STRATEGY
A search for studies on vitamin D, calcium, and periodontitis up to December 2021 was performed in the databases PubMed (MEDLINE, Cochrane Library), Web of Science (WoS), and Scopus. A combination of Medical Subjects Headings (MeSH) and free-text terms were used as a search strategy for each database. The searched terms were: ("vitamin d"[MeSH Terms] OR "calcium"[MeSH Terms]) AND ("periodontitis"[MeSH Terms]); ("vitamin d" OR "calcium") AND ("periodontitis"); TITLE-ABS-KEY (("vitamin d" OR "calcium") AND ("periodont*")). The inclusion criteria were studies with periodontitis patients and controls, with any date or publication language. The exclusion criteria were: a) articles with a relevant risk of bias (score <7 points on the Newcastle-Ottawa methodological quality assessment scale) [8], b) articles with no full-text availability, c) articles without clinical data, and d) studies with non-usable data.
2.2. ASSESSMENT OF METHODOLOGICAL QUALITY
The methodological quality of the articles was screened using the Newcastle-Ottawa (NOS) methodological quality assessment scale composed of eight items that evaluate three dimensions (selection, comparability, and exposure). Considering the score obtained, the studies are classified as high quality (≥7 stars), moderate quality (4-6 stars), and low quality (1-3 stars).
2.3. STATISTICAL ANALYSIS
Data were meta-analyzed with the RevMan 5.4 program (The Cochrane Collaboration, Oxford, UK). For continuous outcomes, the inverse variance (IV) for the mean difference (MD) was used with 95% confidence intervals (95%CI). Heterogeneity was determined according to the Higgins statistic percentage (I2). The random-effects model was applied in case of substantial heterogeneity (I2>50%). A value of P<0.05 was considered the minimum level of significance.
The publication bias risk was assessed with the statistical program MedCalc Statistical Software version 20.027 (MedCalc Software Ltd. Ostend, Belgium), using the funnel plot to assess asymmetry, and the Egger's linear regression test on that a P-value <0.1 suggests the presence of bias.
3. RESULTS
3.1. STUDY SELECTION
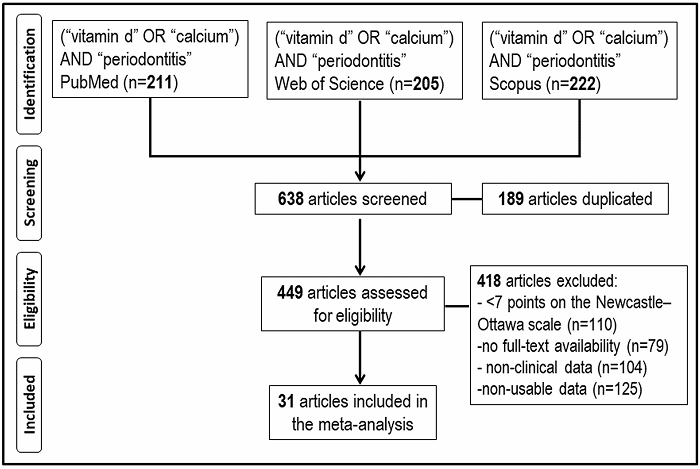
In the initial electronic search, 638 records were found (211 in PubMed, 205 in WoS, and 222 in Scopus) between the years 1972 and 2021. 418 articles were removed based on the exclusion criteria: a) articles with a relevant risk of bias (<7 stars) according to the NOS methodological quality assessment scale (n=110), b) articles with no full-text availability (n=79), c) studies without clinical data (n=104), and d) studies with non-usable data (n=125). After applying these criteria, 31 studies were included in this meta-analysis (Figure 1).
Table 1 shows the main descriptive characteristics and the methodological quality according to the NOS scale of the thirty-one studies [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39] included in this meta-analysis. A total of 14,340 participants with a mean age of 49.5 years (3,268 periodontitis patients and 11,072 controls) were considered. By gender, 1,569 were males (40.13%) and 2,341 females (59.87%).
Table 1: Descriptive characteristics and methodological quality evaluation of the thirty-one articles included in this meta-analysis.
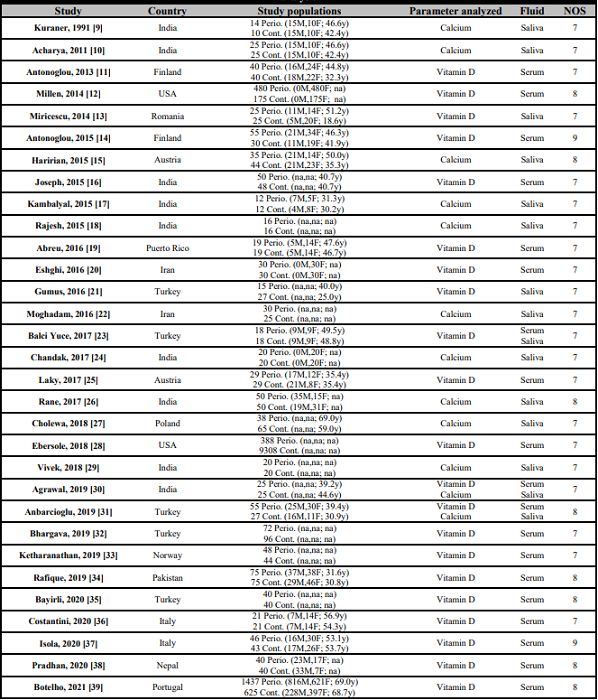
USA: United States of America; Perio.: Periodontitis patients; Cont.: Subjects without periodontitis; M: Male; F: Female; y: Mean age in years; na: Not available data; NOS: Newcastle-Ottawa methodological quality scale.
The screened studies covered fourteen different countries: India (9 studies), Turkey (5 studies), Finland (2 studies), Italy (2 studies), Austria (2 studies), Iran (2 studies), United States of America (2 studies), Puerto Rico (1 study), Poland (1 study), Norway (1 study), Portugal (1 study), Romania (1 study), Nepal (1 study), and Pakistan (1 study). Considering the NOS quality scale, twenty-one articles (67.7%) had 7 stars, eight articles (25.8%) got 8 stars, and two articles (6.5%) reached 9 stars.
3.2. VITAMIN D
The evaluation of serum and saliva vitamin D levels in patients with and without periodontitis is shown in Figure 2. Eighteen studies [11, 12, 14, 16, 19, 20, 23, 25, 28, 30, 31, 32, 33, 34, 35, 37, 38, 39] analyzed serum vitamin D levels (Figure 2a), observing in periodontitis patients, mean vitamin D concentrations 5.88 ng/mL lower than those observed in control subjects without periodontitis. In the statistical analysis, a highly significant relationship was found (MD=-5.88; 95% CI: -8.19 to -3.56; p<0.001).
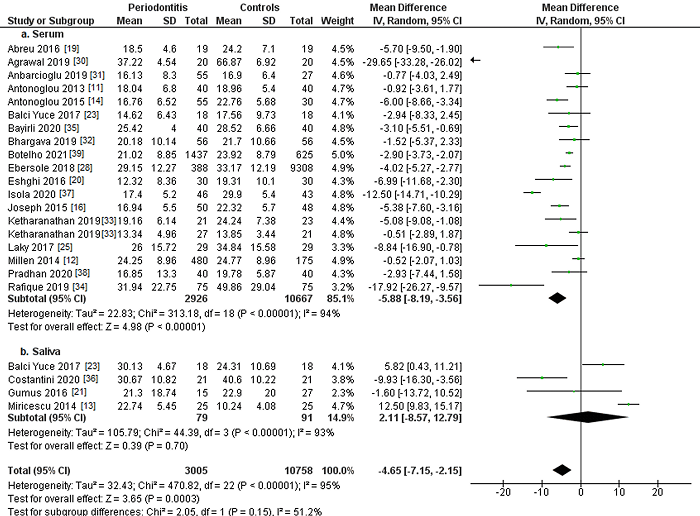
Figure 2: Study data and forest plot graph for serum (a) and salivary (b) vitamin D levels (ng/mL) in periodontitis patients and controls. Mean±SD: mean±standard deviation.
Four studies [13, 21, 23, 36] quantified salivary vitamin D levels (Figure 2b), finding in periodontitis patients mean vitamin D concentrations 2.11 ng/mL higher, although no statistical significance was achieved (MD= 2.11 93% CI: -8.57 to 12.79 p=0.70).
Considering vitamin D concentrations, periodontitis patients had mean concentrations 4.65 ng/mL lower compared to subjects without periodontitis. Statistical analysis showed a highly significant association (MD=-4.65; 95% CI: -7.15 to -2.15; p<0.001).
3.3. CALCIUM
Figure 3 presents the analysis of serum and saliva calcium levels in periodontitis patients and controls. Six studies [9, 15, 24, 27, 30, 31] assessed serum calcium levels in patients with and without periodontitis (Figure 3a). Although periodontitis patients showed 0.01 mg/dL higher mean serum calcium concentrations, the results were not statistically significant (MD=0.01; 95% CI: -0.10 to 0.12; p=0.90).
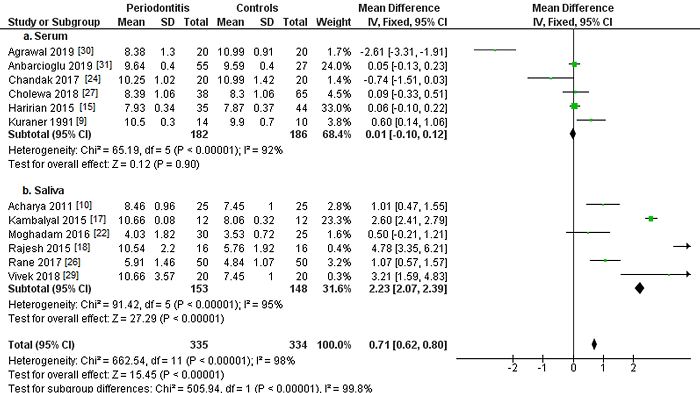
Figure 3: Study data and forest plot graph for serum (a) and salivary (b) calcium levels (mg/dL) in periodontitis patients and controls. Mean±SD: mean±standard deviation.
Another six studies [10, 17, 18, 22, 26, 29] examined salivary calcium levels (Figure 3b), finding mean calcium concentrations 2.23 mg/dL higher in periodontitis patients than in controls. Statistical analysis found highly significant differences (MD=2.23; 95% CI: 2.07 to 2.39; p<0.001).
Considering total calcium levels, periodontitis patients had mean concentrations 0.71 mg/dL higher, with a highly statistically significant association (MD=0.71; 95% CI: 0.62 to 0.80; p<0.001).
3.4. PUBLICATION BIAS
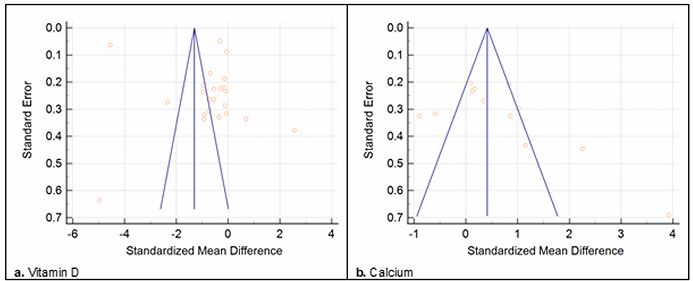
Figure 4 illustrates the publication bias. In the case of vitamin D (Figure 4a), asymmetry is observed in the funnel plot, suggesting publication bias. The results of the Egger test (t=1.54, p=0.02) also showed evidence of publication bias. In the case of calcium (Figure 4b), the funnel plot also presented a non-symmetrical distribution, and Egger's test (t=4.86, p=0.07) confirmed some publication bias.
4. DISCUSSION
In this meta-analysis about the influence of serum and salivary levels of vitamin D and calcium on periodontitis, data from 31 studies have been included.
Vitamin D is essential for the maintenance of periodontal tissues due to its anti-inflammatory effect through the synthesis of antimicrobial proteins and the prevention of bone loss by maintaining bone density homeostasis [40].
In the present study, periodontitis patients had significantly lower mean serum vitamin D concentrations compared to controls (p<0.001). In addition, if the comparison was made differentiating between serum and salivary levels, periodontitis patients showed significantly lower serum vitamin D levels by 5.88 ng/mL (p<0.001) and higher salivary levels although no statistical significance was achieved (p=0.70).
In recent decades, the protective effect of vitamin D on periodontal tissues has been revealed, pointing out vitamin D deficiency as a previous step to periodontal health imbalance [41]. The 21 studies [11, 12, 13, 14, 16, 19, 20, 21, 23, 25, 28, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39] that analyzed this parameter confirmed a higher prevalence of periodontitis in patients with low vitamin D levels.
Bayirli et al. [35] suggest that the protective role of vitamin D against periodontitis is due to the induction of antimicrobial peptides (human beta defensin-2 and cathelicidin). Considering the influence of vitamin D on the oral microbiome, it could decrease the expression of virulence genes of some periodontopathogens such as Porphyromonas gingivalis, and vitamin D deficiency may compromise antimicrobial gene expression, increasing the periodontitis risk [42]. In recent years, there has been a growing interest in the role of vitamin D and calcium as possible etiopathogenic factors in periodontal disease [43]. Numerous studies [19, 23, 28, 30, 35] have shown the relationship between vitamin D levels and periodontitis. For example, Balci Yuce et al. [23] suggest that vitamin D status could be a relevant marker of periodontal bone loss. It would support the potential role of vitamin D in the etiopathogenesis of periodontitis.
Despite the frequency of vitamin D deficiency, especially in the winter, this preventable deficiency could be considered in order to establish more precisely the real role of vitamin D in the etiopathogenesis of periodontal disease. Additional vitamin D intake through diet or supplements could potentially reduce the incidence and severity of periodontal disease [44]. Moreover, this protective effect of vitamin D is greater in women [28]. The link between periodontitis and various systemic diseases such as rheumatoid arthritis [23], cognitive impairment [39], coronary heart disease [37], or type 2 diabetes mellitus [16] could also be related to vitamin D deficiency.
Antonoglou et al. [11] reported a positive relationship between serum 25-hydroxyvitamin D levels and periodontal status in a diabetic population. In addition, they demonstrated that the elimination of the periodontal infectious focus conditioned the increase in serum 25-hydroxyvitamin D levels. Some researchers have highlighted the protective effect of vitamin D for periodontal tissues, indicating the deficiency of this vitamin as a previous step to the loss of periodontal health. Vitamin D develops an anti-inflammatory action due to the secretion of antimicrobial peptides, the inhibition of cytokines or the stimulation of monocytes and macrophages. Likewise, vitamin D avoid an excessive immune response. Serum vitamin D insufficiency could be resulted from the combination of an inadequate intake of foods rich in vitamin D and/or insufficient sunlight exposure, a situation that is increasingly common today due to the reduction of outdoor activities [45].
A case-control study of 60 women demonstrated that those with a serum vitamin D level below 10 ng/mL had a 5.6-fold increased risk of periodontitis compared to those with normal vitamin D levels. When vitamin D levels were in the range of 10-29 ng/mL, this periodontitis risk decreased to 1.46 times [20]. Another study confirmed this association between vitamin D deficiency and periodontal disease. 48.3% of periodontitis patients and only 13.8% of controls had vitamin D deficiency. Periodontitis patients had lower mean serum vitamin D concentrations (18.75 ng/mL) compared to the control group (25.12 ng/mL) [25].
The precise mechanism by which periodontal inflammation affects vitamin D levels or vice versa is still unknown. A possible explanation could be that an amount of serum vitamin D would be used by the host immune cells during periodontal infection [31]. On the other hand, our results do not agree with the findings of a previous prospective study [12] which indicate that plasma vitamin D levels do not alter the course of periodontal disease in a 5-year follow-up. However, the entire population considered in this study was composed only of postmenopausal women.
The existence of a biological plausibility between vitamin D deficiency and periodontitis has been suggested. Adequate vitamin D levels may favourably influence periodontal health due to the regulation of the immune response, the improvement of innate immunity by inducing expression of antimicrobial peptides against periodontopathogens, the anti-inflammatory action, and the maintenance of periodontal bone homeostasis [31].
Other factors that could directly influence vitamin D levels, such as calcium concentrations, have also been studied. In this study, calcium levels in periodontitis patients were significantly higher than in the control group (p<0.01). Of the twelve studies that assessed calcium levels, ten studies [9, 10, 15, 17, 18, 22, 26, 27, 29, 31] found higher calcium levels among periodontitis patients; meanwhile, the remaining two studies [24, 30] did not find them.
Numerous studies have linked high calcium levels with the development of periodontal disease, probably related to the potential mineralization of dental plaque [10, 13, 15, 17, 21, 27, 34, 36]. An increase in salivary calcium levels in conjunction with other factors such as a high salivary pH would reduce the caries risk through the contribution of calcium in the enamel remineralization [36].
However, this calcium availability has a beneficial effect by remineralizing tooth enamel and a detrimental effect by remineralizing dental plaque that could favor the progression of periodontal disease from gingivitis to periodontitis [21].
This mineralized dental plaque increases the retention of bacterial biofilm, which limits oral hygiene and causes gingivitis. The calcified dental plaque may be sufficient to cause periodontitis, despite additional efforts to improve oral hygiene. The mineralized plaque would act as a niche where greater retention and bacterial concentration take place. This situation facilitates gingival tissue inflammation and initiates periodontal disease [18].
Salivary calcium levels increase as periodontal disease progresses from healthy gums to gingivitis and periodontitis. Salivary calcium levels could be used as a biomarker to assess the progression of periodontal disease. Early diagnosis of periodontal disease by measuring salivary calcium levels may help prevent gingivitis or periodontitis with the prompt implementation of periodontal therapies [26].
Instead, Chandak et al. [24] did not find a statistically significant association between Calcium levels and periodontitis, although their entire study sample was menopausal women. Menopause is a condition that decreases hormone levels, especially estradiol, inducing osteoporotic alterations in the periodontal alveolar bone. Other influencing factors on calcium levels should also be considered as tobacco consumption, which independently increases salivary calcium levels by decreasing skeletal bone density [17].
A lower serum vitamin D concentration and a higher salivary calcium concentration are directly associated with an increased risk of periodontitis. However, additional studies conducted in larger populations and with a longer follow-up are required to better understand this relationship.
5. CONCLUSIONS
In this meta-analysis, periodontitis patients showed significantly lower serum vitamin D concentrations than controls without the disease. Vitamin D deficiency could be a factor that promotes periodontitis development.
In addition, periodontitis patients had higher salivary calcium concentrations than controls, favouring the formation of dental plaque and calculus, the main etiological factors of periodontal disease.
6. LIMITATIONS OF THE STUDY
First, the high level of heterogeneity observed in some comparisons may limit the validity and robustness of the quantitative analyses. Second, the influence of the extent and severity of periodontitis on vitamin D and/or calcium levels could not be adequately evaluated. The periodontal status could have been better analyzed using clinically relevant periodontal measures such as probing depth (PD), clinical attachment loss (CAL), or bleeding on probing (BoP). Third, the different methods of quantifying vitamin D levels may have contributed to the heterogeneity. Lastly, the particular characteristics of the study populations or their duration may have influenced the results.