1. INTRODUCTION
In the United States, 21.8% of 75 years or older patients have diabetes [1]. Sodium glucose cotransporter 2 inhibitors (SGLT2i) are the latest antidiabetic treatments that reduce renal glucose reabsorption in the proximal convoluted tubule, leading to increased urinary glucose excretion [2, 3]. EMPA-REG outcome (2015), CANVAS (2017) and DECLARE-TIMI (2019) trials demonstrates the efficacy of SGLT2i in mortality and cardiovascular outcomes and determinate the safety of this pharmacological group, but include patients with a mean age less than 70 years [4, 5, 6]. We conducted this study to analyze tolerability and safety related to SGLT2i in very elderly (> 75 years) T2DM patients.
2. MATERIAL AND METHODS
We conducted a retrospective study of patients treated with SGLT2i in our community (La Rioja) since 2014. All patients older than 75 years (very elderly) were included, excluding those whose follow-up was not performed. Data was collected by the Department of Pharmacy Inspection of the community of La Rioja. Demographic information (age and sex), comorbidities (obesity, diabetes mellitus up to 10 years, target organ damage, hypertension, atrial fibrillation, heart failure, chronic kidney disease, history of cerebrovascular disease and cardiovascular disease) antidiabetic treatment [metformin, repaglinide, sulfonylurea, thiazolinediones, Dipeptidyl peptidase 4 inhibitors (DPP4i), Glucagon-like peptide-1 receptor agonists (GLP1a) and insulin] at the time of inclusion and at 24 months, the type of SGLT2i and the maximum dose were registered. To assess the tolerability of SGLT2i treatment adverse reactions (hypoglycemia, acute kidney injury, ketoacidosis, weight loss, hypotension, urinary and genital infection) and interruption cause were recorded. Values of Hb1Ac, creatinine and glomerular filtrate performed at inclusion, 12 and 24 months were registered. To assess prognosis, mortality during follow-up, HF decompensation (hospital admission and emergency assistance) and cardiovascular diseases during 24 months before SGLT2i treatment were recorded.
Normally distributed continuous variables were expressed as mean±standard deviation (SD). If the variables were not normally distributed, median values with an interquartile range were used. Categorical variables were expressed as numbers and percentages. We analyzed normally distributed continuous variables by Student's t-test, proportions by χ2 test, and continuous variables with skewed distribution by Mann–Whitney test. The strength of the association between the outcome variable (mortality) and each variable considered was assessed by means of odds ratios (ORs) and their 95% confidence intervals (95% CIs). The P-values for all tests were two-sided, and statistical significance was set at P<0.05. Statistical analysis was performed with SPSS version 22 (SPSS, Chicago, IL, USA).
3. RESULTS
Two hundred thirty-five patients treated with SGLT2i were registered. The SGLT2i used in our patients were empagliflozin, in 131 (55.7%), dapagliflozin in 84 (35.7%) and canagliflozin in 20 (8.6%). The mean age was 79.6 ± 3.9 years, 114 were men (48.5%) and 104 were obese (44.3%). The most common comorbidity was hypertension, in 176 patients (74.9%), followed by diabetes mellitus up to 10 years 156 (66.4%), atrial fibrillation in 61 (26%) and heart failure in 49 (20.9%). Fifty-six patients (23.8%) had history of ischemic heart disease and 22 (9.4%) of cerebrovascular disease. Regarding antidiabetic treatment, 184 patients (78.3%) were treated with metformin, 119 (50.6%) with DDP4i, 52 (22.1%) with insulin and repaglinide, 12 (5.1%) with sulfonylurea, 8 (3.4%) with GLP1 agonists and 2 (0.9%) with thiazolinediones. Mean Hb1Ac was 7.9 ±1.4 at the time of inclusion, 7.3 ±1 during the 12 months follow up and 7.3 ± 1.3 at the end of the follow up, showing a decrease in Hb1Ac at the end of the follow-up in 112 (47.7%) patients, with a median decrease of 0.8% (1.4-0.4). The characteristic of patients and according to the iSGLT2 used are shown in Table 1.
Table 1: Characteristic of patients and according to the SGLT2 inhibitor.
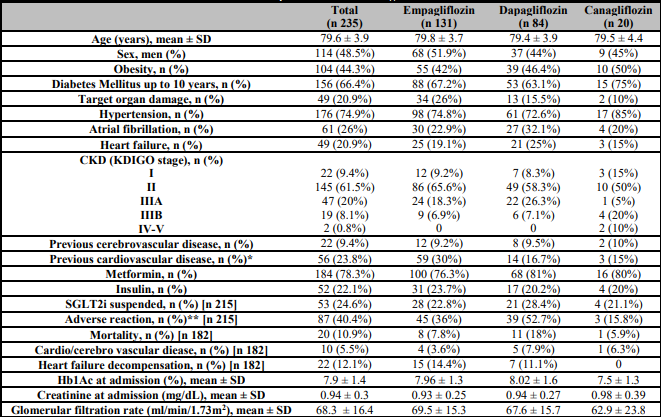
n: number; SD: standard deviation; 0 %: percentage; CKD: Chronic kidney disease; mg/dL: milligram per decilitre; ml: millilitre; min: minute; m2: square meter.
*p < 0.05,
**p <0.01.
All patients were classified in terms of the Kidney Disease: Improving Global Outcomes (KDIGO) classification (Table 2) [7]. Twenty-two patients (9.4%) had normal renal function, 145 (61.7%) had a mild decreased (KDIGO stage 2), 47 (20%) had mild to moderate decrease (KDIGO stage 3a), 19 (8.1%) moderate to severe decrease (KDIGO stage 3b) and only 2 patients (0.8%) had a severely decreased (KDIGO stage 4). Empagliflozin was the most common SGLT2i used in stages 1 to 3b and canagliflozin is the only SGLT2i in stage 4. Albuminuria prior to the start of SLGT2i treatment had been requested in 128 patients (54.4%), being less than 30 mg/g the most common (94 patients). A worsening of initial creatinine and glomerular filtration values (0.94 ± 0.3 and 68.3 ±16.4) was observed during the first year (0.96 ± 0.27 and 66.8 ±16.7), improved at the end of follow-up (0.94 ± 0.27 and 68.2 ±15.8) not related to KDIGO stages. Twenty patients died, 22 presented heart failure decompensation, 5 had an ischemic stroke or transient ischemic attack and 5 an ischemic heart event.
Table 2: Patients classified according to the KDIGO classification.
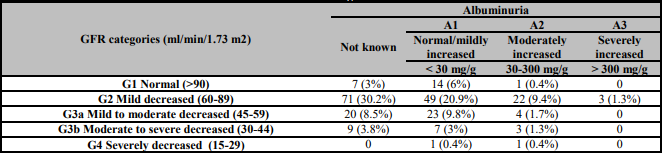
GFR: glomerular filtration rate; mg/g: milligram per gram; ml: millilitre; min: minute; m2: square meter.
During the follow up, 94 adverse events in 84 patients were described, with 53 treatment suspensions. The most common adverse reaction was genitourinary infections (63), followed by acute kidney injury (9), symptomatic hypoglycemia (7) and hypotension (1). Other adverse reactions (14) were cachexia, urinary frequency and weight loss. Adverse reactions were related to sex (59 women vs 28 men, p 0.004), treatment with dapagliflozin (52.7% vs 36% with empagliflozin and 15.8% in canagliflozin, p < 0.001) and mean Hb1Ac at the time of inclusion (8 ± 1.4 vs 7.8 ± 1.3, p 0.04). Genitourinary infections were also more frequent in women (74.6%) and hypoglycemia was related to insulin treatment (10.4% vs 1.2%), age (82.7 ± 5.1 vs 79.4 ± 1.4) and Hb1Ac (9.7 ± 1.4 vs 7.9 ± 1.4) with statistical significance (p < 0.01). The most causes of treatment suspensions were acute kidney injury (8/9), while genitourinary infections (25/63) was uncommon. Six treatment interruptions were produced without adverse reactions.
4. DISCUSSION
Aging may influence the efficacy of SGLT2i due to the decrease in glomerular filtration rate and age-dependent reduction of SGLT2 expression [8]. In the main trials that study the cardiovascular benefits of SGLT2i, less than 10% of the participants were older than 75 years, even knowing that T2DM is a very frequent entity in patients of this age [9, 10, 11]. The efficacy profile and adverse reactions due to SGLT2i do not change with age, not even in elderly (> 65 years old) or very elderly patients [12]. Adverse events were lower in our study compared to those described in the literature, reaching more than 50% with canagliflozin and empagliflozin trials [10, 11]. Several factors influence the increase in genitourinary infections in very elderly patients, such as menopause, urinary retention, prostatic hypertrophy, increased postvoid residual urine, urinary incontinence and increased comorbidities [13]. The most described adverse event was genitourinary infections, with a much higher frequency in our real life study compared to previous trials, but without requiring SGLT2i treatment suspension [9, 10, 11]. On the other hand, the frequency of acute kidney injury and hypoglycemia was lower, not reaching 1% in our study in contrast to previously published, probably due to the low rate of treatment with sulfonylureas and insulin in our patients [9, 10, 11]. Most of the patients showed a glomerular filtration rate greater than 60 ml/min/1.73m2, with a slight worsening of renal function during the first year, improved after two years of treatment, similar to younger patients. The mean age of our patients and the low proportion of adverse effects and treatment suspension of SGLT2i, demonstrate the safety of this pharmacological group in very elderly patients. On the other hand, the Hb1Ac values of our patients were similar to previous trials, with a greater decrease throughout the follow-up, which demonstrates the efficacy of SGLT2i in patients older than 75 years [9, 10, 11].
In conclusion, SGLT2i treatment is effective, safe, and well tolerated in very elderly patients in real life. Despite the fact that genitourinary infections are the most frequent adverse events, they do not imply treatment interruptions.














