Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Angiología
versión On-line ISSN 1695-2987versión impresa ISSN 0003-3170
Angiología vol.75 no.4 Madrid jul./ago. 2023 Epub 27-Nov-2023
https://dx.doi.org/10.20960/angiologia.00483
Originals
Animal model to assess collateral vein sealing with energy-based devices in revascularization surgery
1Angiology, Vascular and Endovascular Surgery Department. Hospital de Manises. Manises, Valencia. Spain
2Instituto de Investigación Sanitaria La Fe. Valencia, Spain
3Angiology, Vascular and Endovascular Surgery Department. Hospital del Mar. Barcelona, Spain
4Angiology, Vascular and Endovascular Surgery Department. Hospital Universitari i Politècnic La Fe. Valencia, Spain
Surgery Department. Faculty of Medicine. Universidad de Valencia. Valencia, Spain
Introduction:
energy sealing devices achieve hemostasis of the vessels through the heat generated and coagulation of the vascular wall proteins. However, the mid-term efficacy peofile for venous graft sealing in arterial bypass surgery remains unknown.
Objectives:
to create an animal model to compare the mid-term efficacy and safety profile at the sealing area after the healing process. To compare and assess which in vivo arterial models show lower morbidity and higher survival rates after 4 weeks.
Material and methods:
this was an in vivo experimental study of 16 New Zealand rabbits. In each rabbit a human saphenous vein (SV) with, at least, 1 venous collateral was implanted. Two arterial models were developed: infrarrenal aorta bypass with SV (n = 5) and aortoplasty with SV patch (n = 11). In both models the collateral was randomized and sealed with either 1 these 2 energy sealing devices: electrothermal Bipolar Vessel Sealing (EBVS) or Harmonic scalpel (HS). Every animal was treated with antithrombotic prophylaxis and immunosuppressive medication. The rates of intraoperative mortality, paraplegia, infection, bleeding, and survival were all studied.
Results:
two animals (50 %) survive 7 days after surgery in the bypass model. However, no animal survived 4 days after surgery in this model. In the aortoplasty group, the 7-day survival rate was 55.56 % (5/9) while the 4-week survival rate was 44.44 % (4/9) (p = 0.05). The rte of paraplegia was 100 % for the bypass model and much lower for the patch group (25 %) (p = 0.03). The mean ischemic time was lower for the aortoplasty model (37.11 min ± 8.1 min) compared to the bypass group (42 min ± 10.61 min) (p = 0.414). No animal showed intrabdominal hemorrhages or adverse drug reactions associated with the immunosuppressive medication.
Conclusion:
aortoplasty with the SV patch model showed lower rates of paraplegia and 7-day mortality in the animal model. Both the bypass and the path model models proved effective at the sealing area. However, only in the aortoplasty model 4 animals survived after 4 weeks.
Keywords: Electrothermal bipolar vessel sealing; Harmonic scalpel; Animal model; Revascularization surgery
INTRODUCTION
Energy-based devices allow safe and effective sealing and closure of blood vessels (1-3). They are widely used in laparoscopic surgery where conventional ligation techniques can extend procedural times (4,5) and even reduce intraoperative bleeding (4,6).
Temperature-controlled bipolar electrocoagulation (BE) and the harmonic scalpel (HS) are among the main energy-based hemostatic devices currently available. BE achieves tissue sealing and coagulation through thermal energy that is generated based on the impedance of clamped tissues and estimated by the instrument itself. It can effectively seal blood vessels with diameters < 7 mm (4).
The harmonic scalpel (HS) achieves hemostasis by utilizing ultrasound energy produced by a piezoelectric material at the tip of the forceps that generates enough thermal energy for tissue coagulation and transection. This device allows the hemostasis of vessels up to 5 mm in diameter (7).
The effectiveness of both devices sealing blood vessels and other tissues is widely recognized in the field of abdominal, thoracic, and cervical surgery based on former studies (3,8,9). In the field of coronary revascularization surgery, these devices have also been used for the endoscopic dissection of the internal saphenous vein or radial artery resulting in good graft patency and a lower rate of wound infection (10,11).
However, in lower limb revascularization surgery, the endoscopic dissection of the saphenous vein assisted by sealing systems with BE has shown inconsistent results. In some of the series published, lower graft patency has been observed possibly associated with the excessive manipulation of the vein with dissection tools (12-14). However, more recent studies have demonstrated that with greater experience in this technique, similar results to those obtained in open safenectomy can be achieved (15,16).
Nevertheless, none of these studies analyze the sealing strength of venous collaterals, and the mid-term efficacy and safety profile of different energy-based hemostatic systems for venous collaterals—subject to high pressure after their arterialization—is unknown to this day.
In a previous study conducted by our group, the efficacy and safety profile of both hemostatic devices were evaluated in venous collaterals of the saphenous vein (SV) in an in vitro model. Venous fragments were interposed in a circuit with a roller pump to simulate pulsatile flow, and pressure inside the system increased gradually until rupture occurred. Bursting pressure was recorded for both devices, and supraphysiological pressures were obtained (788.9 mmHg ± 455 mmHg for BE and 602.5 mmHg ± 363.1 mmHg for HS) without statistically significant differences between them. However, differences were seen in the location of the leakage area. Grafts sealed with BE were more resistant (17).
However, whether resistance of the sealing area after the healing period and the potential risk of aneurysmal degeneration in the graft can lead to mid-term complications is unknown.
To be able to evaluate the characteristics of these sealing devices in vivo, an effective animal model with the highest possible survival rate is necessary. This model should allow us to assess the mid-term efficacy profile of sealing saphenous vein collaterals in an arterial system. With this goal in mind, in this study, we have designed 2 different animal models and analyzed their feasibility in terms of ischemic time, survival, and postoperative complications.
METHODS
Design
This is an in vivo experimental study that used rabbit aortas. Two venous graft models were developed: the first one involved the end-to-end interposition of a segment of a human SV into the infrarenal aorta. The second model, an infrarenal aortoplasty with a patch created out of a human SV with a venous collateral.
The saphenous vein segments were obtained from a cadaver donor or from remnants of revascularization or amputation surgeries.
In both models, dissection of the infrarenal abdominal rabbit aorta was performed followed by the resection of an aortic segment or anterior arteriotomy depending on the model, and the insertion of the human SV graft including a venous collateral. The venous collateral was sealed with either one of the 2 sealing devices—the BE or the HS—based on prior randomization, after aortic unclamping.
In both rabbit aorta models, the animals were sacrificed at 4 weeks, and a resistance test was conducted on the sealing area by catheterizing the aorta and applying distal clamping as well as clamping of its branches. Subsequently, the pressure was progressively increased until rupture occurred in the studied aortic fragment identifying the leak directly or indirectly through pressure loss.
The experimental design was approved by the Animal Experimentation Ethics Committee and the Spanish Department of Agriculture, Environment, Climate Change, and Rural Development, under code 2021/VSC/PEA/0191.
Clinical setting
The extraction of SV fragments from the cadaver donor or remnants of revascularization or amputation surgeries was performed by the Department of Angiology, Vascular and Endovascular Surgery of Hospital Universitari i Politècnic La Fe, Valencia, Spain using routine procedures until a total of 16 SV fragments were obtained. The surgeries performed on the animals, and their postoperative follow-ups and 4-week stabling were conducted at the animal facility of Instituto de Investigación Sanitaria La Fe (IISLAFE), Valencia, Spain under the supervision of personnel in charge and in full compliance with the current applicable regulations.
Subjects
A total of 16 male New Zealand rabbits weighing between 2.5 kg and 3 kg were used for the study. A total of 16 SV fragments were obtained from a cadaver donor or from remnants of revascularization or amputation surgeries.
Variables
The study primary variable was animal survival after the procedure at 4 weeks. Secondary variables included lower limb paresis, presence of abdominal bleeding, and bursting pressure in the resistance test of the sealing area in subjects who survived the 28-day postoperative follow-up period. The following criteria were established as early termination endpoints regarding the experiment: active infection with surgical wound suppuration, peritonitis, uncontrolled pain despite analgesic regimens, signs of cachexia, tachypnea or sustained lethargy, weight loss > 25 %, peritonitis, or fasting periods > 48 hours. In such cases, the animals were euthanized and autopsies performed.
Statistical analysis
A descriptive analysis of the variables was conducted for each animal model group. Non-parametric analyses (Wilcoxon-Mann-Whitney test) were used to compare parameters such as procedural time, ischemia time, and bursting pressures. The non-parametric Fisher's exact test was used to compare dichotomous variables like mortality and postoperative paraplegia. All analyses were performed using SPSS statistical software package (version 26.0 or higher).
PROCEDURE
Surgical technique
All animals were anesthetized by veterinary personnel from IISLAFE. Preoperative medication included intravenous (IV) enrofloxacin 10 mg/kg, IV metoclopramide 0.5 mg/kg, IV ranitidine 2 mg/kg to prevent gastric ulcers, and IV meloxicam 0.25 mg/kg as an analgesic drug. Anesthesia was induced using intramuscular (IM) ketamine (50 mg/kg) and IM xylazine (5 mg/kg). Prior to the administration of drugs, the animal was immobilized by wrapping it with a cloth and injecting the premedication into the femoral biceps. Afterwards, the ear marginal vein was cannulated, and general anesthesia was maintained using 2.5 % to 3 % sevoflurane and IM boluses of fentanyl-fluanisone 0.2-0.3 ml/kg every 30 to 45 min.
The surgical technique consisted of a midline laparotomy approach, exposure of the retroperitoneum, and dissection of the infrarenal aorta and lumbar branches. After systemic heparinization with a bolus of sodium heparin (0.5 mg/kg of body weight), the infrarenal aorta was clamped immediately above the common iliac arteries. A fragment of the human SV was interposed according to the animal aorta models suggested.
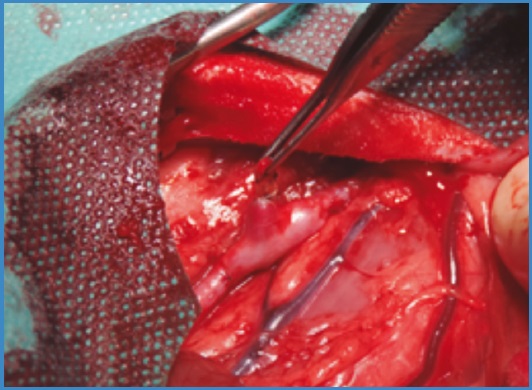
In the first model described an aorto-aortic bypass was performed with the interposition of a venous fragment of approximately 3 cm with the collaterals under study (Fig. 1). The grafts were end-to-end sutured with both proximal and distal anastomoses being performed using continuous 6/0 polypropylene sutures with × 2.5 magnification.
In the second model described, an infrarenal abdominal aortoplasty was performed using a venous fragment with the collaterals to be sealed (Fig. 2). Continuous suturing of the graft was performed using the same material described above.
After completing the vascular sutures, the clamps were removed following prior flushing with 1 % heparinized saline solution. Afterwards, the venous collateral was sealed using one of the devices (BE or HS). Ligasure® (Maryland Sealer/Divider, Covidien, CO, United States) and the Valleylab® LS10 generator were used for BE sealing while the Sonicision® device (Cordless Ultrasonic Dissector, Covidien, CO, United States) was used for the HS. The integrity of the sealing was immediately checked after unclamping.
Hemostasis of the surgical site was verified, and the abdominal wall closed in layers using continuous sutures of 3/0 polyglycolic acid for the deeper tissues and continuous intradermal sutures with 4/0 polyglycolic acid for the skin.
Postoperative follow-up
The animals remained in individual cages and maintained in accordance with the current legislation regarding the care of animals used in experimentation and with other scientific purposes, as outlined in the Spanish Royal Decree 53/2013, Annex II, Section 2.
For the first 3 days, meloxicam was administered subcutaneously (sc) once every 24 hours, metoclopramide (sc) every 12 hours until intestinal transit was restored, and ranitidine (sc) every 12 hours as a gastric protector. Buprenorphine was used for postoperative pain control at doses of 0.03 mg/kg (sc) every 12 hours. Dandelion hay was provided ad libitum to prevent cecal impaction, facilitate intestinal peristalsis, and prevent stasis.
All animals transitioned to a normal diet 24 hours after the procedure, and oral cyclosporine was administered at doses of 5 mg/kg every 24 hours to prevent graft rejection.
Euthanasia
In surviving animals, euthanasia was performed at 4 weeks following the protocols anticipated by the legislation. An overdose of pentobarbital was administered followed by exsanguination after resection of the study specimen (abdominal aorta with experimental SV graft) (Fig. 2) preceded by sedation with ketamine and xylazine.
Resistance test of the sealing area
This test was conducted in animals that survived the 4-week postoperative period. To run this test, the rabbit's infrarenal abdominal aorta was catheterized through midline laparotomy followed by clamping of both iliac arteries and lumbar branches. Euthanasia was then performed followed by a gradual increase of intra-arterial pressure with an Encore® manometer syringe (Boston Scientific, United States) until rupture and leakage occurred in the sealing area of the collateral or in the aortic wall. Bursting pressure was recorded using a GOD0002 industrial manometer (Goodyear®, United States).
RESULTS
The first aorta model (end-to-end interposition of an SV graft into the infrarenal aorta) was initially used as the technique of choice in the first 5 rabbits. However, 1 animal died while anesthesia was being induceed. The individual results for each subject are shown on table IA.
Table IA. Results and individual characteristics of experimental animals with the interposition graft model.
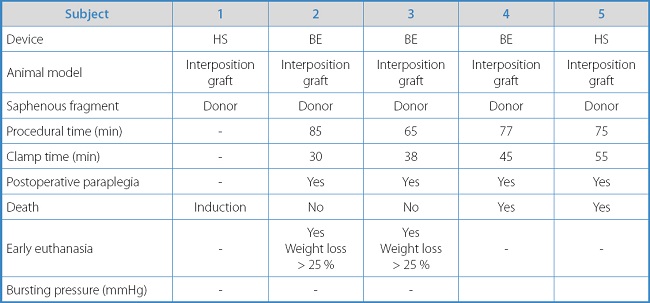
The mean procedural time for the aorto-aortic bypass model was 75 min ± 8.23 min, with a mean ischemia time of 42 min ± 10.61 min.
The survival rate in this group at 7 days was 50 % (2 subjects). The rate of paraplegia in all rabbits operated using this model was 100 %. In the 2 surviving rabbits, early euthanasia had to be performed 3 weeks after surgery due to a loss of animal welfare due to weight decrease > 25 % following paraplegia. No intra-abdominal hemorrhage was reported in the autopsies of the 2 spontaneously deceased rabbits or in the 2 rabbits that were euthanized. In all the cases presented, both the sealing of the collateral and patency of the aorto-iliac sector were confirmed.
In this animal model, the resistance test of the sealing area could not be performed or bursting pressure was obtained as no animal survived the necessary 4-week postoperative period to complete the healing process.
After considering the possible causes of mortality and the higher rate of paraplegia than expected, experimentation was interrupted to reassess the protocol and refine the surgical procedure. Among the different alternative models analyzed to minimize peri-aortic dissection and clamp time, the model of infrarenal aortoplasty with SV patch was eventually the chosen one. With this model, the remaining 11 rabbits were operated on. The individual results for each subject are shown on table IB.
Table IB. Results and individual characteristics of experimental animals with the aortoplasty model.
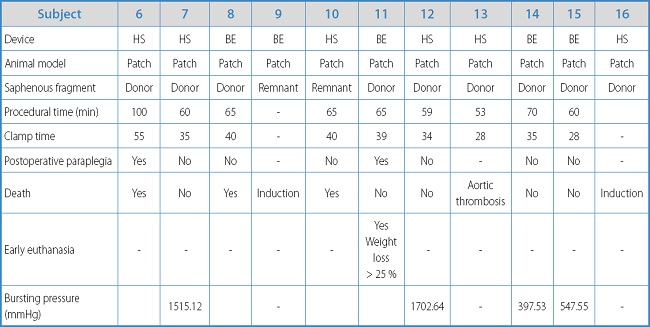
In this model, the mean procedural time was 66.33 min ± 13.53 min, which was shorter compared to the interposition graft model (p = 0.076). The mean ischemia time was 37.11 min ± 8.1 min, shorter compared to the aorto-aortic bypass model (42 min ± 10.61 min), but without statistically significant differences (p = 0.414).
The 7-day survival rate was 5 out of 9 animals (55.56 %), as there were 2 losses due to preoperative mortality during the induction of anesthesia. The rate of paraplegia was 25 %, which is significantly lower compared to the interposition graft model (100 %) (p = 0.03). The 4-week survival rate was 4 out of 9 animals (44.44 %). In 1 of the animals, early euthanasia had to be performed 7 days after the surgery due to a loss of animal welfare, and significant weight loss due to paraplegia. In all autopsies, intra-abdominal bleeding was ruled out with presence of fibrotic reaction around the collateral sealing area.
In the 4 survivors, a resistance test of the sealing area was run with mean rupture pressure for the BE device (n = 2) of 491.29 mmHg ± 132.59 mmHg and mean bursting pressure for the HS sealing system (n = 2) of 1608.88 mmHg ± 132.59 mmHg without statistically significant differences being reported.
Regarding the location of the leakage, for BE, in 1 case it occurred at the sealing area and in the other case at the aortic lateral wall while for the HS, 1 burst occurred at the area sealed by the device, and the other at the connections of the pressure recording system at 1515 mmHg.
No infectious complications or other adverse events to immunosuppression were reported in any of groups studied.
DISCUSSION
The use of energy-based sealing devices is effective to achieve hemostasis in arteries or veins in procedures like nephrectomy (8), hysterectomy (18) or thoracotomy (19). However, different bursting pressures have been reported between venous and arterial territories with venous pressures being lower (20). Therefore, studies are needed to assess the efficacy profile of sealing in the mid-term in venous collaterals undergoing high-pressure systems, as in the case of lower limb revascularization surgery.
In a former study conducted by our group, non-inferiority of these sealing systems compared to conventional ligation was demonstrated in venous collaterals undergoing arterial pressures and with supraphysiological bursting pressures in an in vitro hemodynamic circuit (17).
However, to demonstrate their durability and, therefore, their mid-term efficacy profile, it's necessary to demonstrate their performance in an in vivo model that allows us to assess resistance to rupture in collaterals under physiological conditions once the usual inflammatory and healing periods have been met beyond the 3-week mark (21).
The rabbit aorta model is the best one thanks to its anatomical characteristics in its abdominal section with mean length and diameters (3 cm × 3.7 mm), which is consistent with segments of the ISV. Additionally, this model is cheaper and easier to use and maintain compared to the alternative canine carotid artery model.
In the study conducted by Ribas et al. (22), a polydimethylsiloxane graft was implanted in New Zealand rabbits as a prosthetic bypass model in small-sized arteries. In that study, the survival rate was 46.9 %, and the paraplegia rate, 17.2 % in survivors.
In our study, the end-to-end aortic interposition graft model had a 50 % mortality rate at 7 days and a 100 % mortality rate at 4 weeks, as well as a 100 % paraplegia rate. This high rate of neurological complications due to spinal cord ischemia resulted in a postoperative period with more comorbidities due to difficulties moving the animal, lack of spontaneous urination that requiring daily emptying maneuvers, and progressive weight loss > 25 %. All these factors led to a loss of animal welfare and, therefore, the fulfillment of the endpoint criteria for euthanasia purposes.
A similar rate of complications has been described in other experimental studies with rabbits as a model of spinal cord ischemia. In the study conducted by Saba et al. (23), 37 rabbits underwent endoluminal aortic clamping for 30 min to create a model of spinal cord ischemia. In the group without neuroprotection, the rate of paraplegia was 100 % while in the intra-arterial diltiazem group while the rate of paraparesis was 12.5 % (Tarlov score < 3) at 48 hours. In the study conducted by Oyar et al. (24), the experiment was performed with 27 rabbits with rates of paraplegia of 100 % in the group without neuroprotection and 75 % at 48 hours in the adrenomedullin group. The severity of paraparesis was greater in animals with ischemia time > 30 min, which explains the higher rate of neurological complications associated with time (23,24).
Unlike the study conducted by Ribas et al. (22) where the arterial model consisted of an aortic bypass and a new prosthetic material with 2 anastomoses in an end-to-end configuration to allow tissue reperfusion after the first anastomosis, our first model involved end-to-end anastomosis with straight interposition of the venous graft resulting in a longer ischemia time due to clamping of the aorta during both vascular sutures. This may partially justify the higher rates of mortality and paraplegia described.
Given the high morbidity and mortality rates reported with this model, and following the principle of refinement in laboratory animals, a second animal model was proposed involving an infrarenal aortoplasty with a human saphenous vein graft. This new model required 1 vascular anastomosis only and, therefore, a shorter spinal cord ischemia time. This, added to the fewer number of lumbar arteries that required temporary occlusion during the ischemic period, resulted in lower rates of paraplegia (25 %) in the surviving subjects.
The anatomy of the rabbit's arteries is similar to the human arteries in terms of the bifurcation of the abdominal aorta into common iliac arteries and the presence of lumbar branches (25). However, in rabbits, the lumbar arteries have limited collateral circulation, thus contributing to more neurological complications during manipulation and temporary occlusion of the infrarenal aorta and its branches (26).
The rabbit has 7 pairs of lumbar arteries all of which originate from the aorta posterior wall. The seventh pair is often the distal branch of the median sacral artery that, in 50 % of the cases, is caudal to L6. Aortic bifurcations are found in 53.3 % of cases and are cranial to L7 in rabbits (27). This means that there is limited space for dissection at distal infrarenal aortic level if all lumbar branches are to be spared to prevent spinal cord ischemia.
In the second model, which involves a shorter clamped length of the aorta and only 1 anastomosis, a lower rate of paraplegia was seen. Additionally, the aortoplasty was performed as caudally as possible to spare the median sacral artery from which the last pair of lumbar arteries arise. In cases where the arterial ostium was located more caudally, reflux was controlled by placing 2 opposing 3/0 silk ligatures without circumferentially enclosing each artery as shown on figure 3. This reduced the reflux of the median sacral artery and allowed venous graft anastomosis in the aorta under optimal conditions.

Figure 3. Intraoperative image of middle sacral artery with reflux control using a pair of 3/0 silk ligatures without completely encircling the artery to prevent injuries.
Both in the arterial interposition graft and in the aortoplasty model, effective hemostasis was demonstrated with no evidence of abdominal bleeding in all autopsies. However, only in the latter arterial model survivors were obtained after 4 weeks to run the resistance test of the sealing area. Bursting pressure with both devices was always supraphysiological with mean pressures of 491.29 mmHg for BE and 1608.88 mmHg for HS. However, no statistically significant differences were found.
In all autopsies, patency of the aortoiliac sector treated was confirmed except 1 case where early aortic thrombosis was found during the procedure and early euthanasia was decided based on the predetermined endpoint criteria. No bleeding or intra-abdominal hemorrhage were identified, and the sealing area remained intact in both the interposition graft and aortoplasty models.
As study limitations we should mention that the sample size was small for the bursting pressure test. Despite an initial sample size of 16 animals, the test could only be run on 4 rabbits. One of the main limitations was the low survival rate in the end-to-end bypass animal model. Nevertheless, the bursting pressure obtained was always supraphysiological (> 300 mmHg). However, no significant differences were found between the 2 devices due to the insufficient number of subjects used to run the resistance test.
In future studies, the animal model should be refined to obtain more survivors after the 4-week healing process. Although rabbits have a higher risk of spinal cord ischemia, they are considered the most suitable animal model due to their similar aortic diameter compared to the saphenous vein and because they meet the reduction criterion of avoiding using larger species, which would involve more technical difficulties from the veterinary postoperative care standpoint, as well as a series of bio-ethical implications.
This preliminary study allowed us to fine tune the protocol to assess the efficacy profile of sealing venous collaterals using energy-based devices with acceptable mortality and paraplegia rates in an in vivo rabbit aorta model.
However, despite partially addressing the challenge of early spinal cord ischemia, an insufficient number of subjects was obtained to allow proper comparisons between the 2 devices regarding bursting pressure due to overpressure. Therefore, in the coming future, the study will continue with the development of this second aorta model until a sufficient number of survivors is achieved to verify the mid-term efficacy of collateral sealing using energy-based systems.
Falcón Espínola M, Plana Andani E, Bisbal Velasco V, Clarà Velasco A, Miralles Hernández M. Animal model to assess collateral vein sealing with energy-based devices in revascularization surgery. Angiología 2023;75(4):218-225
REFERENCES
1. Rajbabu K, Barber NJ, Choi W, et al. To knot or not to knot? Sutureless haemostasis compared to the surgeon's knot. Ann R Coll Surg Engl 2007;89(4):359-62. DOI: 10.1308/003588407X183418 [ Links ]
2. Lacin T, Batirel HF, Ozer K, et al. Safety of a thermal vessel sealer on main pulmonary vessels. Eur J Cardiothorac Surg 2007;31(3):482-5. DOI: 10.1016/j.ejcts.2006.11.038 [ Links ]
3. Luo Y, Li X, Dong J, et al. A comparison of surgical outcomes and complications between hemostatic devices for thyroid surgery: a network meta-analysis. Eur Arch Otorhinolaryngol 2017;274(3):1269-78. DOI: 10.1007/s00405-016-4190-3 [ Links ]
4. Toishi M, Yoshida K, Agatsuma H, et al. Usefulness of vessel-sealing devices for ≤7 mm diameter vessels: a randomized controlled trial for human thoracoscopic lobectomy in primary lung cancer. Interact Cardiovasc Thorac Surg 2014;19(3):448-55. DOI: 10.1093/icvts/ivu176 [ Links ]
5. Ding Z, Wable M, Rane A. Use of Ligasure bipolar diathermy system in vaginal hysterectomy. J Obstet Gynaecol 2005;25(1):49-51. DOI: 10.1080/01443610400024609 [ Links ]
6. Richter S, Kollmar O, Schilling MK, et al. Efficacy and quality of vessel sealing: comparison of a reusable with a disposable device and effects of clamp surface geometry and structure. Surg Endosc 2006;20(6):890-4. DOI: 10.1007/s00464-005-0380-6 [ Links ]
7. Clements RH, Palepu R. In vivo comparison of the coagulation capability of SonoSurg and Harmonic Ace on 4 mm and 5 mm arteries. Surg Endosc 2007;21(12):2203-6. DOI: 10.1007/s00464-007-9345-2 [ Links ]
8. Leonardo C, Guaglianone S, de Carli P, et al. Laparoscopic nephrectomy using Ligasure system: preliminary experience. J Endourol 2005;19(8):976-8. [ Links ]
9. Lamberton GR, Hsi RS, Jin DH, et al. Prospective comparison of four laparoscopic vessel ligation devices. J Endourol 2008;22(10):2307-12. DOI: 10.1089/end.2008.9715 [ Links ]
10. Van Linden A, Hecker F, Lehmann-Grube J, et al. Randomized Trial of 2 Endoscopic Radial Artery Harvesting Devices-Immunofluorescence Assessment. Ann Thorac Surg 2020;110(3):897-902. DOI: 10.1016/j.athoracsur.2019.12.063 [ Links ]
11. Perrault LP, Kollpainter R, PagéP, et al. Techniques, complications, and pitfalls of endoscopic saphenectomy for coronary artery bypass grafting surgery. J Card Surg 2005;20(4):393-402. DOI: 10.1111/j.1540-8191.2005.SCS13.x [ Links ]
12. Jauhari YA, Hughes CO, Black SA, et al. Endoscopic vein harvesting in lower extremity arterial bypass: a systematic review. Eur J Vasc Endovasc Surg 2014;47(6):621-39. DOI: 10.1016/j.ejvs.2014.02.009 [ Links ]
13. Wartman SM, Woo K, Herscu G, et al. Endoscopic vein harvest for infrainguinal arterial bypass. J Vasc Surg 2013;57(6):1489-94. DOI: 10.1016/j.jvs.2012.12.029 [ Links ]
14. Santo VJ, Dargon PT, Azarbal AF, et al. Open versus endoscopic great saphenous vein harvest for lower extremity revascularization of critical limb ischemia. J Vasc Surg 2014;59(2):427-34. DOI: 10.1016/j.jvs.2013.08.007 [ Links ]
15. Kronick M, Liem TK, Jung E, et al. Experienced operators achieve superior patency and wound complication rates with endoscopic great saphenous vein harvest compared with open harvest in lower extremity bypasses. J Vasc Surg 2019;70(5):1534-42. DOI: 10.1016/j.jvs.2019.02.043 [ Links ]
16. Khan SZ, Rivero M, McCraith B, et al. Endoscopic vein harvest does not negatively affect patency of great saphenous vein lower extremity bypass. J Vasc Surg 2016;63(6):1546-54. DOI: 10.1016/j.jvs.2016.01.032 [ Links ]
17. Falcón Espínola M, Requejo García L, Plana Andani E, et al. Evaluación in vitro de los dispositivos de energía para el sellado de colaterales de vena safena en cirugía de revascularización. Angiología 2019;71(1):2-10. DOI: 10.20960/angiologia.00016 [ Links ]
18. Elhao M, Abdallah K, Serag I, et al. Efficacy of using electrosurgical bipolar vessel sealing during vaginal hysterectomy in patients with different degrees of operative difficulty: a randomised controlled trial. Eur J Obstet Gynecol Reprod Biol 2009;147(1):86-90. DOI: 10.1016/j.ejogrb.2009.07.011 [ Links ]
19. Tsunezuka Y, Waseda R, Yachi T. Electrothermal bipolar vessel sealing device LigaSureV for pulmonary artery ligation--burst pressure and clinical experiences in complete video-assisted thoracoscopic major lung resection for lung cancer. Interact Cardiovasc Thorac Surg 2010;11(3):229-33. DOI: 10.1510/icvts.2010.239087 [ Links ]
20. Hruby GW, Marruffo FC, Durak E, et al. Evaluation of surgical energy devices for vessel sealing and peripheral energy spread in a porcine model. J Urol 2007;178(6):2689-93. DOI: 10.1016/j.juro.2007.07.121 [ Links ]
21. Broughton GI, Janis JE, Attinger CE. Wound Healing: An Overview. Plastic and Reconstructive Surgery 2006;117(7S):1e. DOI: 10.1097/01.prs.0000222562.60260.f9 [ Links ]
22. Ribas LM, Torres IO, Appolonio F, et al. Experimental implantation of an arterial substitute made of silicone reinforced with polyester fabric in rabbits. Clinics 2017;72(12):780-4. DOI: 10.6061/clinics/2017(12)10 [ Links ]
23. Saba T, Manduz S, Sapmaz I, et al. Neuroprotective effects of diltiazem in rabbits with occluded aorta. Rev Bras Cir Cardiovasc 2007;22(4):416-24. DOI: 10.1590/S0102-76382007000400007 [ Links ]
24. Oz Oyar E, Korkmaz A, KardesşO, et al. Aortic cross-clamping-induced spinal cord oxidative stress in rabbits: the role of a novel antioxidant adrenomedullin. J Surg Res 2008;147(1):143-7. DOI: 10.1016/j.jss.2007.06.025 [ Links ]
25. Balastegui MT, Ramos-PláJJ, Ferrer-Puchol MD, et al. Anatomical variations in the aortic bifurcation in new zealand white rabbits on arteriography. Anat Rec (Hoboken) 2014;297(4):663-9. DOI: 10.1002/ar.22874 [ Links ]
26. Mazensky D, Radonak J, Danko J, et al. Anatomical study of blood supply to the spinal cord in the rabbit. Spinal cord 2011;49(4):525-8. DOI: 10.1038/sc.2010.161 [ Links ]
27. Mazensky D, Flesarova S, Sulla I. Arterial Blood Supply to the Spinal Cord in Animal Models of Spinal Cord Injury. A Review. Anat Rec (Hoboken) 2017;300(12):2091-106. DOI: 10.1002/ar.23694 [ Links ]
Received: November 13, 2022; Accepted: December 26, 2022











 texto en
texto en