Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Archivos de Zootecnia
versão On-line ISSN 1885-4494versão impressa ISSN 0004-0592
Arch. zootec. vol.60 no.232 Córdoba Dez. 2011
https://dx.doi.org/10.4321/S0004-05922011000400032
Origin of Cuban Creole cattle inferred by patri- and matrilineages
Inferencia del origen del bovino Criollo Cubano a través del análisis de patri- y matrilinajes
Lirón, J.P.1, Acosta, A.2, Rogberg-Muñoz, A.1, Uffo, O.2, Posik, D.M.1, García, J.3, Peral García, P.1 and Giovambattista, G.1*
1Instituto de Genética Veterinaria (IGEVET). CCT La Plata. Facultad de Ciencias Veterinarias. Universidad Nacional de La Plata. CONICET. La Plata. República Argentina. *ggiovam@fcv.unlp.edu.ar
2Laboratorio de Genética Molecular. Centro Nacional de Sanidad Agropecuaria. San José de las Lajas. La Habana. Cuba.
3Dirección Nacional de Genética. MINAGRI. Cuba.
This research was supported by Fondo Argentino de Cooperación Horizontal (FOAR) del Ministerio de Relaciones Exteriores, Comercio Internacional y Culto de la República Argentina (Proyecto FO-AR No: 5724), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Ministerio de Agricultura, Cuba. Universidad Nacional de La Plata, and Instituto Nacional de Investigación.
SUMMARY
Cattle was absent from America before the discovery. Initially, bovine were brought to Greater Antilles (La Española, Puerto Rico, Jamaica and Cuba islands), and in the course of a few years, they were taken from Caribbean islands to the rest of Latin America. Nowadays, Cuban Creole cattle population is about 1300 heads, mainly located in the eastern region of the island. With the aim of analyzing the maternal origin of Cuban Creole cattle and detect possible contemporaneous, male mediated, gene flow, a 240 pb fragment of mitochondrial D-loop (mtDNA) and five microsatellites of Y chromosome (BTY) were studied in 36 dams and 21 sires, respectively. Genetic diversity was evaluated through number of haplotypes, mean number of pairwise differences and nucleotide diversity. The phylogenetic analysis was performed using a median joining. A total of 15 mtDNA haplotypes were detected in the studied population (10 from the European haplogroup T3, 3 from the African T1, 1 from the Nearern East T2, and 1 ambiguous T1-T3). The number of polymorphic sites, the mean nucleotide diversity, and the mean number of pairwise differences were 23, 0.014 and 3.36, respectively. Two patrilinages were detected, both belonging to the Y3 Zebu haplogroup. In conclusion, Cuban Creole cattle population had a mtDNA haplotypic composition similar to the observed in Creole and Mediterranean breeds, what is in concordance with its historical origin. Y chromosome analysis evidenced a male mediated process of zebu introgression.
Key words: Mitochondrial DNA. Y Chromosome. Polymorphism.
RESUMEN
Antes de descubrimiento, no existían bovinos en América. Los primeros, fueron introducidos en la Antillas Mayores (La Española, Puerto Rico, Jamaica y Cuba), y desde allí trasladados al resto de Latinoamérica. Actualmente, existen en Cuba alrededor de 1300 bovinos Criollos, concentrados principalmente en la región oriental. Con el objetivo de analizar el origen materno de esta raza y detectar eventos contemporáneos de flujo génico por vía paterna, se analizó un fragmento de 240 pb del D-loop mitocondrial (mtADN) y 5 microsatélites del cromosoma Y (BTY), en 36 hembras y 21 machos respectivamente. La diversidad genética se estimó mediante el número de haplotipos, el número de sitios polimórficos, el número de diferencias nucleotídicas entre pares de secuencias y el índice de diversidad nucleotídica, mientras que el análisis filogenético se realizó utilizando el método de median joining network. Dicho análisis permitió detectar 15 haplotipos mitocondriales (10 del haplogrupo europeo T3, 3 del africano T1, 1 del cercano oriente T2 y 1 ambiguo T1-T3) y 3 haplotipos en el BTY, ambos del haplogrupo cebuíno Y3. En el mtADN se detectaron 23 sitios polimórficos con una diversidad nucleotídica de 0,014 y 3,36 diferencias medias entre pares de secuencias. En conclusión, la población de bovinos Criollos Cubanos presentó una composición haplotípica mitocondrial comparable a la de otras razas criollas y mediterráneas, hecho que concuerda con su origen histórico. El BTY evidenció altos niveles de introgresion paterna de genes del zebú.
Palabras clave: ADN mitocondrial. Cromosoma Y. Polimorfismo.
Introduction
Anthropological, paleontological and historical evidences show that cattle was absent from America before the discovery. First bovines were brought by Spanish settlers in 1493 (Primo, 1992), and during the next 50 years, Creole cattle founder populations were established by Spanish and Portuguese settlers. Initially, cows were brought to Greater Antilles (La Española, Puerto Rico, Jamaica and Cuba islands), and in the course of a few years, they were taken from Caribbean islands to Central and South America and to the south of the modern United States. Simultaneously, there were direct shipments of Portuguese cattle to Brazil (De Alba, 1978, Primo, 1992). In only few decades, the Creole cattle spread over Latin America, being the only bovine for more than 300 years until the introduction of selected European and Indian breeds.
Nowadays, a number of distinct local Creole breeds are found throughout the Americas (Wilkinset al., 1982; Primo, 1992). This cattle shows great phenotypic heterogeneity and is adapted to a wide range of environments, with a moderate human intervention. In Cuba, Creole cattle, the descendant of bovine introduced into the island by Spanish settlers during the first years of the colonization, are named Cuban Creole. It has been suggested that Cuban Creole is related to the Spanish breeds Rubia Gallega, Asturiana, and Andaluza (Uffo, 2003). Furthermore, Cuban Creole was influenced by animals, with Bos indicus blood, introduced from Jamaica. This Zebu introgression was studied by cytogenetic and microsatellite analysis (Sánchez et al., 1977; Uffo et al., 2006). Cuban Creole is a hump-less bovine (Bos taurus) with horns; and is double purpose breed (meat and milk), and also an exceptional working animal. It has been adapted to the Cuban tropical environment for over 500 years. Due to its high adaptability and versatility, are of great importance in this Cuban region. Even though, the conservation of this breed has been affected by the introduction of foreign breeds, especially due to economical aspects (Uffo, 2003). Cuban Creole cattle suffered a severe reduction in population size during the 20th century as a result of the introduction and massive production of highly selected European and Zebu commercial breeds. As a consequence, the distribution of Creole populations which originally covered most of Cuban territory became mainly restricted to eastern region of the Island. Currently, the most abundant dairy and beef breed in Cuba are Cuban Zebu, Brahman, Siboney (5/8 Holstein and 3/8 Cuban Zebu) and Holstein; while there are around 1320 heads of female Creole cattle mainly located in the eastern region of the island (DNG, 2010).
Mitochondrial DNA (mtDNA) and Y chromosome DNA uniparental markers and autosomal markers (DNA sequences, microsatellites, AFLP, SNPs) have been widely and successfully used to study the origins of domestic cattle as well as breed relationships (Bruford et al., 2003; Giovambattista et al., 2010). Although the mtDNA, Y chromosome and autosonal markers are informative, they account for different events of the population history. In this sense, mtDNA and Y chromosome analysis retrieve exclusively the genetic matrilineal and patrilineal relationships, respectively. In consequence, they have been a valuable tool, aimed to phylogeographical studies, to understand the origin and domestication of livestock. In the particular case of Creole cattle studies, Y chromosome markers have a powerful resolution to detect contemporaneous gene flow, because of the predominant asymmetric mating occurring during the last century, between Creole dams and foreign sires, to improve livestock (Giovambattista et al., 2000; Lirón et al., 2006b, 2008; Ginja et al., 2010).
MtDNA control region polymorphisms have been reported in American Creole cattle breeds from several North, South, Central and Caribbean countries (Magee et al., 2002; Miretti et al., 2002, 2004; Carvajal-Carmona et al., 2003; Mirol et al., 2003; Lirón et al., 2006a; Ginja et al., 2010). These studies reported the existence of European T3, African T1, and Middle Eastern T2 haplotypes. African haplotypes in America, were also identified as belonging to two different sub-haplogroups, T1* and T1a (AA1 in Miretti et al., 2002 nomenclature; Mirettiet al., 2002, 2004; Lirónet al., 2006a). In addition, the patrilinages present in Creole cattle were analyzed through Y chromosome microsatellites and single nucleotide polymorphisms (SNPs) (Giovambattista et al., 2000; Ginja et al., 2010). The aim of the present work was to analyze mitochondrial and Y chromosome polymorphisms in Cuban Creole cattle to investigate the maternal origins and possible contemporaneous male mediated gene flow. Results of this analysis are discussed in the context of previous genetic and historical data obtained in Creole breeds.
Material and methods
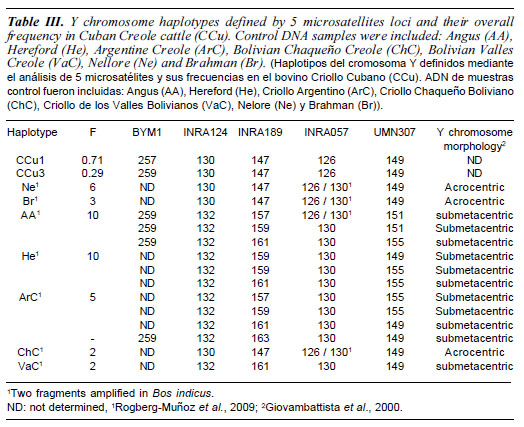
A total of 36 Cuban Creole dams were sampled from the eastern region of Cuba for matrilineage analyses. Additionally, 21 sires, that represent the whole set of males from the Artificial Insemination Centre from Cuban National Direction of Genetics, were sampled for patrilineage analysis. All studied animals were phenotypically hump-less bovine (Bos taurus) with horns and tancoloured. Available pedigree information was used to select individuals in order to sample the whole genetic diversity in Cuban Creole cattle.To check Taurine or Zebu Y chromosome lineage haplotypes, data from 38 DNA samples [Angus (AA)= 10, Hereford (He)= 10, Argentine Creole (ArC)= 5, Bolivian Chaqueño Creole (ChC)= 2, Bolivian Valles Creole (VaC)= 2, Nellore (Ne)= 6, Brahman (Br)= 3,], were included. These samples have been previously analysed for Y chromosome morphology (acrocentric or submetacentric) by conventional cytogenetic methods and genotyped for Y chromosome STRs (Giovambattista et al., 2000; Rogberg-Muñoz et al., 2009).
The mtDNA hypervariable region I of the control region (nucleotide positions 16023-16262 in the complete mitochondrial genome sequence, Accession No. V00654; Anderson et al., 1982) was sequenced using primers L15960 and H16334 as described by Troy et al. (2001). Amplification products were purified with polyethylene glycol 8000 and sequenced in an automatic DNA sequencer MegaBACE 1000 (GE Healthcare) by using DYEnamic ET Terminator Kit (GE Healthcare) and PCR primers. Raw sequences were edited by using Sequence Analyzer (GE Healthcare).
Five Y chromosome microsatellites (BYM1, INRA124, INRA189, INRA057 and UMN307A (table I) were amplified in two multiplex in a total volume of 12.5 ml using a DNA Engine Thermal Cycler (Bio-Rad Laboratories Inc.). Each reaction contained: 1X reaction buffer with 1.5 mM of MgCl2 provided by the supplier, 0.2 mM of each dNTP, 0.25 U Taq polymerase (Metabion International AG, Martinsried, Germany), 0.2 to 0.8 µM of each primer, and 40 ng of DNA. The cycling conditions were: a denaturation step of 2 min at 94oC, followed by 10 cycles of 1 min at 94 oC, 45 sec at 60oC, and 45 sec at 72oC, and followed by 25 cycles of 1 min at 94oC, 45 sec at 58, and 45 sec at 72oC, with a final elongation step of 15 min at 72 oC. For BYM1, annealing temperatures were 66oC in the first 10 cycles and 64oC in the next 25 cycles. Fragments were separated by electrophoresis in an automatic DNA sequencer MEGABACE 1000 (GE Healthcare, USA) and analyzed with MegaBase fragment profiler (version 1.2; GE Healthcare). ET-ROX 550 was used as internal size standard (GE Healthcare).
Variations in the D-loop region were defined by direct comparison with the reference bovine mtDNA sequence (Accession No.V00654, Andersonet al. (1982). Number of mtDNA and BTY haplotypes were calculated directly, while mean number of pairwise nucleotide differences (Tajima, 1993) and nucleotide diversity (Nei, 1987; Tajima, 1993) were calculated using the algorithms implemented in Arlequin 3.5 (Schneider et al., 2000; http://cmpg.unibe.ch/software/arlequin3), using the default general setting. A median joining network was constructed using the methodology described in Bandelt et al. (1995) using Network 4.516 (http://www.fluxus-technology.com/sharenet.htm, consulted 06/01/10), using default setting and the same weight (10) for transitions and transversion. The nucleotide sequences obtained were compared by applying blastn 2.2.24 against cattle D-loop DNA sequences previously reported in the GenBank database.
Results and discussion
D-loop analysis in 36 Cuban Creole dams showed a total of 23 polymorphic sites characterizing 15 haplotypes (table II). These polymorphisms included 21 transitions and two transversions by comparison with the bovine reference sequence (Anderson et al., 1982). As expected, haplogroup T3, the most prevalent in Western Europe, was also the most commonly found with a incidence of 69.4%. This haplogroup was represented by ten haplotypes that diverge from the consensus sequences in one or two mutations. The African haplogroup T1 was found 8 times (22.2%), and comprised 3 haplotypes. Remarkably, one T1 haplotype has 3 out of the 4 mutations that defined the sub-haplogroup T1a. Haplogroup T2, which is frequently observed in the Near East with a minor presence in European breeds, was detected in only two individuals (5.5%) (figure 1). The remaining haplotype is ambiguous because it has only two of the three T1 diagnostic sites (T at position 16050 and C at position 16113). None of the analyzed Cuban Creole cattle presented Zebu maternal haplotypes. The mean nucleotide diversity was 0.014 (SD 0.008) and the mean number of pairwise differences was 3.36 (SD 1.76). All sequences have been submitted to GenBank (accession numbers FJ611967-FJ611986, FJ799719FJ799722, FJ857930-FJ857932, and HM448433-HM448440). Regarding Y chromosome results, only three patrilineages were detected, all belonging to Zebu Y3 type (table II).
From a maternal perspective, Cuban Creole cattle shared the main characteristics previously reported for Creole and Mediterranean breeds. In this sense, the mitochondrial diversity was 100% taurine, and it was composed mainly by T3 haplogroup, followed by T1 haplogroup, and T2 haplogroup represented at low frequency. The consensus T3 haplotype, predominant in Europe (including the Iberian Peninsula), was the most frequent in this native breed (36.1%). Furthermore, a considerable percentage of private sequences (specific allele of a population/breed) for Cuban (four out of fifteen) or American Creole cattle (one out of fifteen) was found. The remaining eight haplotypes, excluding the T3 and T1 nodal sequences, have a wide geographical distribution in Europe, all of them were detected in Iberian breeds, including South Spanish and Portuguese ones like Retinta, Mostrenca, Berrenda. Furthermore, several of these haplotypes were also shared with other Creole cattle populations, including the T2 haplotype previously detected in Colombia. Outstandingly, Cuban population shared haplotypes with neighbouring Creole breeds distributed in the Minor Antilles (Antigua, Guadalupe, St. Lucia); Central and south of North America (Mexico and USA), and northern countries of South America (Colombia and Ecuador). In contrast, they have only one haplotype in common with Creole breeds from the maridional countries of South America (Argentine, Bolivia, Brazil, Paraguay and Uruguay). None of detected haplotypes, with the exception of T3 and T1 nodal sequence, was shared with Canarian Islands native breeds, despite these islands were a common intermediate port used by Spanish ships. Within T1 haplogroup, Cuban Creole cattle presented a haplotype displaying had three out of the four mutations that define the T1a subhaplo-group, mainly characteristic of Brazil and Minor Antilles. Despite T1a haplogroup has not been yet detected in Africa, intermediate haplotypes between T1 nodal and T1a have also been identified in Tunisian and Southern Portugal breeds (Beja-Pereira et al., 2006; Ginja et al., 2010).
Different explanations have been proposed for the geographical origin of African lineages in American Creole cattle. Carvajal-Carmona et al. (2003) explained the presence of haplogroup T1* in Colombia as originating from North Africa, probably as the result of the Arab occupation of Iberia prior to European migration to the New World; while Magee et al. (2002) suggested that the African influence in Creole is, at least in part, attributable to the direct historical importation of West African cattle to the Caribbean. Miretti et al. (2004) postulated that haplogroup T1a (AA1 in their nomenclature) would have originated in Spain, while T1* would have arrived in America from West Africa. By contrast, an alternative hypothesis suggested the possibility of introgression of at least part of the African mtDNA haplotypes (including the T1a) from somewhere in mainland Africa, perhaps following the slave trade routes (Lirón et al., 2006a, 2008). In conclusion, the African haplogrupes present in Cuban Creole cattle, as well as in other Creole populataions, could be introduced directly from Iberia and alternatively following slave trading route.
Regarding Y chromosome results, the studied population exhibited two haplotypes, both belonging to Zebu Y3 haplogroup despite Cuban Creole is phenotypically hump-less cattle. Taurine or Zebu haplotype origin was defined by INRA124 and INRA057. This genetic diversity of patrilineages seems to be low compared with other species, such as human. However, previous works in bovine and other domestic animals, showed a similar reduced number of within-breed haplotypes observed at high frequency (Li et al., 2007; Ginjaet al., 2009, 2010; Kantanen et al., 2009; Pérez-Pardal et al., 2009). This situation is even more extreme in highly selected breeds where one haplotype is often fixed or close to be fixed. This low level of patrilineages is probably related to the reduced effective male population size characteristic of domestic animal species, as well as intensive breeding programs and artificial insemination practice. Different studies have been published using different genetic markers and different primers, in consequence microsatellites molecular sizes and haplotypes are difficult to be compare without standardization. Even though, the haplotypes detected in Cuban Creole cattle could correspond to any of the haplotypes H1 to H11 (INRA124*130 - INRA189*88 BYM1*256) reported by Liet al. (2007), and/ or H18Y3 to H21Y3 (INRA124*130 - INRA189*88 - UMN307*149) reported by Ginja et al. (2010). Several Latin American Creole cattle reflect a high degree of Zebu introgression mediated by males. It is notable that these introgressions appear to be higher in tropical and lowland regions, than in temperate and highland cattle (Giovambattista et al., 2000; Lirón et al., 2006b). Cuban Creole Y chromosome variation is in agreement with the cytogenetic study performed by Sánchez et al. (1977), which shows the precence of Zebu acrocentric Y chromosome. Furthermore, the historical data propose that the cattle arrived from Jamaica to Cuba had Bos indicus influence. The introduction of Zebu animals could have increased the degree of Zebu introgression during the last century. In concordance patrilineage data, Uffo (2003) and Uffo et al. (2006) found high frequencies of Zebu-specific alleles in microsatellites and milk proteins (CASA1C, CASBA, LAAB) study of this population.
In conclusion, Cuban Creole cattle population present a pattern of genetic admixture between Taurine and Zebu. Discrepancies are observed between mitochondrial and Y chromosome markers, with a Taurine mtDNA haplotypic composition comparable with that observed in other Creole and Mediterranean breeds (as well as European), while Y chromosome evidenced a probably recent male mediated process of Zebu introgression. These different pictures are in concordance with the historical origin of this breed. The genetic characterization, using molecular markers, of indigenous breeds, such Cuban Creole cattle, it is useful and necessary information for define the conservation units and it is one of the previous recommended steps for design conservation plan of these important reservoirs of genetic diversity for commercial domestic species (Delgado Bermejo et al., 2010).
References
Anderson, S., de Bruijn, M.H., Coulson, A.R., Eperon, I.C., Sanger, F. and Young, I.G. 1982. Complete sequence of bovine mitochondrial DNA. J. Mol. Biol., 156: 683-717. [ Links ]
Bandelt, H.J., Forster, P., Sykes, B.C. and Richards M.B. 1995. Mitochondrial portraits of human populations using median networks. Genetics, 141: 743-753. [ Links ]
Beja-Pereira, A., Caramelli, D., Lalueza-Fox, C., Vernesi, C., Ferrand, N., Casoli, A., Goyache, F., Royo, L.J., Conti, S., Lari, M., Martini, A., Ouragh, L., Magid, A., Atash, A., Zsolnai, A., Boscato, P., Triantaphylidis, C., Ploumi, K., Sineo, L., Mallegni, F., Taberlet, P., Erhardt, G., Sampietro, L., Bertranpetit, J., Barbujani, G., Luikart, G. and Bertorelle G. 2006. The origin of European cattle: Evidence from modern and ancient DNA. Proc. Natl. Acad. Sci. USA, 103: 8113-8118. [ Links ]
Bruford, M.W., Bradley, D.G. and Luikart G. 2003. DNA markers reveal the complexity of livestock domestication. Nat. Rev. Genet., 4: 900-910. [ Links ]
Carvajal-Carmona, L.G., Bermudez, N., Olivera-Angel, M., Estrada, L., Ossa, J., Bedoya, G. and Ruiz-Linares A. 2003. Abundant mtDNA diversity and ancestral admixture in Colombian Criollo cattle (Bos taurus). Genetics, 165: 1457-1463. [ Links ]
De Alba, J. 1978. Progress in the selection of the Latin American dairy Criollo. World. Anim. Rev. FAO, 28: 26-30. [ Links ]
Delgado Bermejo, J.C., Martínez Martínez, A., Camacho Vallejo, M.E. y Vega Pla, J.L. 2010. Conservación de razas de especies domésticas. En: Giovambattista, G. y Peral-García, P. (Eds.). Genética de animales domésticos. Inte-Médica. Buenos Aires. Argentina. 272 pp. [ Links ]
DNG, 2010. Balance de razas puras 2009. Dirección Nacional de Genética. Ministerio de la Agricultura. La Habana. Cuba. [ Links ]
Ginja, C., Telo da Gama, L. and Penedo, M.C. 2009. Y chromosome haplotype analysis in Portuguese cattle breeds using SNPs and STRs. J. Hered. 100: 148-157. [ Links ]
Ginja, C., Penedo. M.C., Melucci, L., Quiroz, J., Martínez López, O.R., Revidatti, M.A., Martínez-Martínez, A., Delgado, J.V. and Gama, L.T. 2010. Origins and genetic diversity of New World Creole cattle: inferences from mitochondrial and Y chromosome polymorphisms. Anim Genet., 41: 128-141. [ Links ]
Giovambattista, G., Ripoli, M.V., Luca, J.C. De, Mirol, P., Lirón, J.P. and Dulout, F.N. 2000. Geographic distribution and frequency of taurine Bos taurus and zebu B. indicus Y chromosome incidence amongst Argentine and Bolivian Creole cattle breeds. Anim. Genet., 31: 302-305. [ Links ]
Giovambattista, G., Lirón, J.P., Bravi, V. It, C., Prando, A. y Peral García, P. 2010. El aporte de la genética en la elucidación de la historia de la domesticación y diferenciación de las especies domésticas. En: Giovambattista, G. y Peral-García, P. (Eds.). Genética de animales domésticos. Inte-Médica. Buenos Aires. Argentina. 272 pp. [ Links ]
Kantanen, J., Edwards, C.J., Bradley, D.G., Viinalass, H., Thessler, S., Ivanova, Z., Kiselyova, T., Cinkulov, M., Popov, R., Stojanoviæ, S., Ammosov, I. and Vilkki, J. 2009. Maternal and paternal genealogy of Eurasian taurine cattle (Bos taurus). Heredity, 103: 404-415. [ Links ]
Li, M.H., Zerabruk, M., Vangen, O., Olsaker, I. and Kantanen J. 2007. Reduced genetic structure of north Ethiopian cattle revealed by Y-chromosome analysis. Heredity, 98: 214-218. [ Links ]
Lirón, J.P., Bravi, C.M., Mirol, P.M., Peral-García, P. and Giovambattista, G. 2006a. African matrilineages in American Creole cattle: evidence of two independent continental sources. Anim. Genet., 37: 379-382. [ Links ]
Lirón, J.P., Peral-García, P. and Giovambattista, G. 2006b. Genetic characterization of Argentine and Bolivian Creole cattle breeds assessed through with microsatellites. J. Hered., 97: 331-339. [ Links ]
Lirón, J.P., Posik, D.M.,MacNeil, M.D. , Mirol, V.A., Rogberg-Muñoz, A., Martínez-Martínez, A., Maciel, S., Terán-Polo, C.R., Peral-García, P., Hanotte, O. y Giovambattista, G. 2008. Origen histórico y afinidad filogeográfica de los bovinos criollos americanos a través del estudio del ADN mitocondrial. IX Simposio Iberoamericano sobre Conservación y Utilización de Recursos Zoogenéticos. Mar del Plata. Buenos Aires. Argentina. 10-12 Diciembre. pp. 311-315. [ Links ]
Liu, W.S., Mariani, P., Beattie, C.W., Alexander, L.J. and Ponce de León, F.A. 2002 A radiation hybrid map for the bovine Y Chromosome. Mamm. Genome., 13: 320-326. [ Links ]
Kappes, S.M., Keele, J.W., Stone, R.T., McGraw, R.A., Sonstegard, T.S., Smith, T.P., Lopez-Corrales, N.L. and Beattie, C.W. 1997 A secondgeneration linkage map of the bovine genome. Genome Res., 7: 235-249. [ Links ]
Magee, D.A., Meghen, C., Harrison, S., Troy, C.S., Cymbron, T., Gaillard, C., Morrow, A., Maillard, J.C. and Bradley, D.G. 2002. A partial African ancestry for the Creole cattle populations of the Caribbean. J. Hered., 93: 429-432. [ Links ]
Miretti, M.M. Jr., Pereira, H.A., Poli, M.A., Contel, E.P.B. and Ferro, J.A. 2002. African-derived mitochondria in South American native cattle breeds (Bos taurus): Evidence of a new taurine mitochondrial lineage. J. Hered., 93: 323-330. [ Links ]
Miretti, M.M., Dunner, S., Naves, M., Contel, E.P. and Ferro, J.A. 2004. Predominant Africanderived mtDNA in Caribbean and Brazilian Creole cattle is also found in Spanish Cattle (Bos taurus). J. Hered., 95: 450-453. [ Links ]
Mirol, P.M., Giovambattista, G., Lirón, J.P. and Dulout, F.N. 2003. African and European mitochondrial haplotypes in South American Creole cattle. Heredity, 91: 248-254. [ Links ]
Nei, M., 1987 Molecular evolutionary genetics. Columbia University Press. New York, NY. USA. [ Links ]
Pérez-Pardal, L., Royo, L.J., Beja-Pereira, A., Curik, I., Traoré, A., Fernández, I., Sölkner, J., Alonso, J., Álvarez, I., Bozzi, R., Chen, S., Ponce de León, F.A. and Goyache, F. 2009. Y-specific microsatellites reveal an African subfamily in taurine (Bos taurus) cattle. Anim. Genet., 41: 232-241. [ Links ]
Primo, A.T. 1992. El ganado bovino ibérico en las Américas: Quinientos años después. Arch. Zootec., 41: 421-432. [ Links ]
Rogberg-Muñoz, A., Ripoli, V., Villegas-Castagnasso, V., Posik, D., Peral-García, P. and Giovambattista, G. 2009. Male Wagyu lineage origin in crossbred steers through Y chromosome DNA. TRACE 5th Annual Meeting and Conference. Munich. Alemania, 1-3 abril. Book of abstracts. pp. 40. [ Links ]
Sánchez, A., Betancourt, A. y Gutiérrez, C. 1977. Cromosomas del ganado Criollo. Congreso Panamericano de Veterinaria y Zoonosis. Colombia. [ Links ]
Schneider, S., Roessli, D. and Excoffier, L. 2000. Arlequin: A software for population genetics data analysis. Ver. 2.0. Genetics and Biometry Laboratory. Department of Anthropology. University of Geneva. [ Links ]
Tajima, F. 1993. Measurement of DNA polymorphism. In: Takahata, N. and Clark, A.G. (Eds.). Mechanisms of molecular evolution. Introduction to molecular paleopopulation biology. Japan Scientific Societies Press, Sinauer Associates. Sunderland, MA. Inc. Tokyo. pp. 37-59. [ Links ]
Troy, C.S., MacHugh, D.E., Bailey, J.F., Magee, D.A., Loftus, R.T., Cunningham, P., Chamberlain, A.T., Sykes, B.C. and Bradley, D.G. 2001. Genetic evidence for Near-Eastern origins of European cattle. Nature, 410: 1088-1091. [ Links ]
Uffo, O. 2003. Aplicación de los marcadores moleculares al estudio de la biodiversidad del ganado bovino cubano. Tesis de Doctorado. Universidad Agraria de la Habana. www.censa.edu.cu/produccioncientifica/tesis (18/06/10). [ Links ]
Uffo, O., Martín-Burriel, I., Martínez, S., Ronda, R., Osta, R., Rodellar, C. and Zaragoza P. 2006. Caracterización genética de seis proteínas lácteas en tres razas bovinas cubanas. Boletín AGRI-FAO, 39: 15-24. [ Links ]
Vaiman, D., Mercier, D., Moazami-Goudarzi, K., Eggen, A., Ciampolini, R., Lépingle, A., Velmala, R., Kaukinen, J., Varvio, S.L., Martin, P., Levéziel, H. and Guérin, G. 1994. A set of 99 cattle microsatellites: characterization, synteny mapping, and polymorphism. Mamm. Genome, 5: 288-297. [ Links ]
Ward, T.J., Skow, L.C., Gallagher, D.S., Schnabel, R.D., Nall, C.A., Kolenda, C.E., Davis, S.K., Taylor, J.F. and Derr, J.N. 2001. Differential introgression of uniparentally inherited markers in bison populations with hybrid ancestries. Anim. Genet., 32: 89-91. [ Links ]
Wilkins, J.V., Martinez, L. y Rojas, F. 1982. El ganado vacuno Criollo. CIAT. Santa Cruz, Bolivia. Documento 31. [ Links ]
Recibido: 18-6-10
Aceptado: 8-2-11.