Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Archivos de Zootecnia
versión On-line ISSN 1885-4494versión impresa ISSN 0004-0592
Arch. zootec. vol.61 no.234 Córdoba jun. 2012
https://dx.doi.org/10.4321/S0004-05922012000200016
SHORT NOTE
Synergistic effects of insulin-like growth factor (IGF-II) and FSH on bovine granulosa cells
Efectos sinérgicos del factor de crecimiento similar a la insulina (IGF-II) y la FSH sobre células de la granulosa bovina
Smith, O.F.1*, Ogunsola, A.A.1, Ladokun, A.O.1, Ajadi, T.A.2 and Onagbesan, O.M.1
1Department of Animal Physiology. University of Agriculture. Abeokuta. Nigeria. *smith_olusiji@yahoo.com
2Department of Veterinary Surgery. University of Agriculture. Abeokuta. Nigeria.
SUMMARY
The synergistic effects of insulin-like growth factor II (IGF-II) and follicle stimulating hormone (FSH) and the effect of IGF-II alone on progesterone and estradiol production by bovine granulosa cells (GC) cultured in vitro were determined. Granulosa cells obtained from ovaries of slaughtered cows were cultured for four days in carbon (IV) oxide incubator at 37oC, 5% CO2 in atmospheric air and 100% relative humidity, using Tissue Culture Medium 199 (TCM 199) as culture medium. The medium was changed on the second day and testosterone added to serve as substrate for estradiol production. IGF-II alone, or IGF-II (10 ng/ mL)+ FSH was added to culture medium at 0, 0.1, 1.0, 10, 50, and 100 (ng/mL) levels of inclusion. Concentrations of progesterone and estradiol produced were measured by radioimmunoassay. Data collected was statistically analysed using analysis of variance method. The results obtained showed that IGF-II alone and IGF-II + FSH had significant effects (p<0.05) on progesterone and estradiol produced by cultured GC. From the result obtained, it can be concluded that IGF-II + FSH gave better synergistic effects on bovine granulosa progesterone and estradiol production.
Key words: Granulosa cells culture. Steroid hormones.
RESUMEN
Se estudiaron los efectos sinérgicos del factor de crecimiento II similar a la insulina (ICF-II) solo o asociado a hormona folículo estimulante (FSH), sobre la producción de progesterona y estradiol por las células de granulosa bovinas (GC) cultivadas in vitro. Las GC, obtenidas de ovarios de vacas sacrificadas, fueron cultivadas durante cuatro días en incubador óxido de carbono (IV) a 37oC y 5% de CO2 en aire atmosférico y 100% de humedad relativa, usando el medio de cultivo de tejidos 199 (TCM 199). El medio, fue cambiado al segundo día y se añadió testosterona como sustrato para la producción de estradiol. El IGFII solo o IGF-II (10 ng/mL) + FSH, se añadieron a las proporciones de 0; 0,1; 1,0; 10; 50 y 100 (ng/mL). Las concentraciones de progesterona y estradiol producidos se midieron mediante radioinmunoensayo. Los datos obtenidos se sometireron a análisis de varianza. Los resultados obtenidos demuestran que el IGF-II, solo o IGF-II + FSH tuvieron efectos significativos (p<0,05) sobre la progesterona y el estradiol producidos por las GC cultivadas. La asociación IGF-II + FSH mostró efecto sinérgico sobre la producción, de progesterona y estradiol por la granulosa bovina.
Palabras clave: Cultivos de células de granulosa. Hormonas esteroides.
Introduction
The insulin-like growth factors (IGFs) are members of a family of low molecular weight of single-chain polypeptide named for their structural and functional similarity to insulin (Strauss and Barbieri, 2009). The stimulatory effects of insulin, IGF-I and -II on estradiol production by mammalian granulosa cells are documented (Guidice, 1992) and are likely due in part to their ability to enhance the action of gonadotropins on ovarian follicular steroidogenesis (Spicer et al., 2002). The IGFs enhance FSH actions in vitro, including stimulation of estradiol production (Spicer and Echternkamp, 1995) by granulosa cells of multiple species including cattle (Minegishi et al., 2000). In cattle, evidence indicates that IGFs play an important regulatory role once follicles reach the antral stage and become gonadotropin dependent (Monget and Bondy, 2000). Consequently, this study aimed to evaluate the effects of preincubated granulosa cells of ovaries obtained from local abattoir with IGF-II and FSH on steroid hormones production.
Materials and methods
The study was conducted at the Department of Animal Physiology, College of Animal Science and Livestock Production, University of Agriculture, Abeokuta, Nigeria. Ten pairs of ovaries were collected from the local abattoirs and were kept in 0.9% normal saline containing 100 mg/mL streptomycin sulfate and 100 IU/mL Penicillin-GSodium. They were transported tothe laboratory within one hour after slaughter.
PREPARATION OF CULTURE MEDIA
The medium used for the cell culture was TCM-199 (Sigma Aldrich Chemical Co., USA). It was diluted in 1.0 litre of de-ionized water and 3.36 mM of NaHCO3 was added to each litre of TCM-199 solution. Also, 1% Bovine Serum Albumin (BSA) was then added to a half of the TCM-199 while 5% Fetal Calf Serum (FCS) was added to 47.5 mL from the remaining part of TCM-199 while 0.8% antibiotics was also added to each half of the medium, after which 0.2 µm Supor® membrane filter was used to filter both media for the purpose of sterility.
METHODOLOGY
Immediately returning to the laboratory, ovaries were washed in fresh sterile saline. All visible follicles were aspirated into the culture medium. Granulosa cells collected were separated from follicular fluid by centrifuging at 3000 rpm for 10 min. Cell pellet were then mixed thoroughly with the culture medium and aliquoted at 50 µl/mL per well into 24-well micro plates containing 450 µL of medium per well. Cultures were incubated at 37oC in a 95% air and 100% relative humidity. Media was changed after 2 days of incubation and cells were maintained in the presence of 10% fetal calf serum for the first 48 hours of culture. Testosterone (10 ng/mL each) was added as substrate for estradiol production at the third day of the culture. On the 4th day, the experiment was completed.
DATA COLLECTION
At the termination of the experiment, cultured medium samples was collected for determination of estradiol and progesterone concentration by Radioimmunoassay method. Samples were packaged and sent to the Laboratory for Physiology and Immunology, Department of Animal Science, Katholeic University of Leuven, Belgium. Hormone was assayed in maturation media according to Abraham et al. (1972).
EXPERIMENTAL DESIGN
The experimental design included: (1) Supplementation of additives; i.e. (a) addition of IGF-II and FSH; (b) IGF-II alone, and varied levels of inclusion: (a) 0 ng/mL, (b) 0.1ng/mL, (c) 1.0 ng/mL, (d) 10 ng/mL, (e) 50 ng/mL and (f) 100 ng/mL. Each treatment was replicated 3 times and data collected was statistically analysed by analysis of variance. Significant means were separated using Duncan Multiple Range Test (Duncan, 1955).
Results
IGF-II EFFECTS ON BOVINE GRANULOSA CELLS CULTURED IN VITRO FOR STEROIDS PRODUCTION
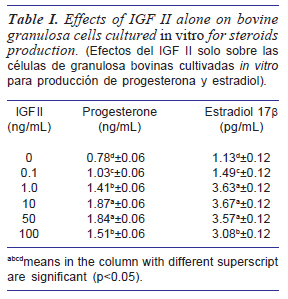
Table I showed the effects of IGF-II on bovine granulosa cells cultured in vitro for estradiol production. The highest concentration of progesterone was recorded at 10 ng/mL level of IGFII. Meanwhile, the results of IGF-II effects on estradiol production showed no significant differences among 1 ng/mL, 10 ng/mL and 50 ng/mL levels of inclusion.
SYNERGISTIC EFFECTS OF IGF-II WITH FSH ON BOVINE GRANULOSA CELLS CULTURED IN VITRO FORSTEROIDS PRODUCTION
The combination of IGF-II (at 10 ng/mL) with FSH inclusion in culture medium for bovine granulosa cells culture has significant effect on the progesterone production as shown in table II (p<0.05). FSH inclusion level at 50 ng/mL recorded the highest value of progesterone (5.11 ng/mL). It could be deduced from the same table that there was significant difference in the value of estradiol recorded by varying levels of IGFII and FSH combination.
Discussion
The results obtained in this study showed a significant effect on progesterone and estradiol production - a pointer to the proliferation of GC. This is validated by the report of Lowe (1991) that IGF-II stimulates uptake of both amino acids and glucose, and acts as progression factor in the cell cycle. It was also reported by Savion et al. (1981) that in addition to the known metabolic effects of insulin and IGFs, they have been shown to stimulate both progesterone production and mitosis of bovine ovarian granulosa cells cultured in vitro. In vivo data also compliment the result of this study by revealing a positive correlation between follicular fluid IGF-II and progesterone concentration in postpartum anestrous and cyclic cows (Echternkampet al., 1990; Spicer and Enrighgt, 1991). Some studies revealed that IGF acts on the GC to augment hormonal actions of gonadotropins (Veldhuis and Rodgers, 1987; LaVoie et al., 1999). Veldhuis and Rodgers (1987) suggested that the synergism between FSH and IGF augmented rates of progesterone synthesis by activating dual mechanisms: enhancement of effective cellular uptake and utilization of low-density lipoprotein (LDL)-borne sterol substrate and stimulation of functional cholesterol side chain cleavage activity. It can be concluded that, gonadotropin and IGFII significantly influenced the steroidogenic activity of bovine GC.
Acknowledgement
The authors thanked members of the Laboratory for Physiology and Immunology, Department of Animal Science, Katholeic University of Leuven, Belgium for the Radioimmunoassay analysis.
References
Abraham, B.M., Ketterson, J.B. and Roach, P.R. 1972. Magnetic field dependence of the adiabatic susceptibility tensor of powdered cerum magnesium nitrate. Phys Rev B, 6: 4675. [ Links ]
Duncan, D.B. 1955. Multiple range and multiple F tests. Biometrics, 11: 1-42. [ Links ]
Echternkamp, S.E., Spicer, L.J., Gregory, K.E., Canning, S.F. and Hammond, J.M. 1990. Concentrations of insulin-like growth factor-I in blood and ovarian follicular fluid of cattle selected for twins. Biol Reprod, 43: 8-14. [ Links ]
Guidice, L.C. 1992. Insulin-like growth factors and ovarian follicular development. Endocr Rev, 13: 641-669. [ Links ]
LaVoie, H.A., Garmey, J.C. and Veldhuis, J.D. 1999. Mechanisms of insulin-like growth factor I augmentation of follicle-stimulating hormoneinduced porcine steroidogenic acute regulatory protein gene promoter activity in granulosa cells. Endocrinology, 140: 146-153. [ Links ]
Lowe, W.L. 1991. Biological actions of the insulinlike growth factors. In: LeRoith, D. (Ed.). Insulinlike growth factors: Molecular and cellular aspects. CRC Press. Boca Raton. pp. 49-95. [ Links ]
Minegishi, T., Hirakawa, T., Kishi, H., Abe, K., Abe, Y., Mizutani, T. and Miyamoto, K. 2000. A role of insulin-like growth factor I for follicle-stimulating hormone receptor expression in rat granulosa cells. Biol Reprod, 2: 325-333. [ Links ]
Monget, P. and Bondy, C. 2000. Importance of the IGF system in early folliculogenesis. Mol Cell Endocrinol, 163: 89-93. [ Links ]
Savion, N., Lui, G., Laherty, R. and Gospodarowicz, D. 1981. Factors controlling proliferation and progesterone production by bovine granulosa cells in serum-free medium. Endocrinology, 109: 409. [ Links ]
Spicer, L.J. and Enrighgt, W.I. 1991. Concentrations of insulin-like growth factor 1 and steroids in follicular fluid of preovulatory bovine ovarian follicles: Effect of daily injections of a growth hormone-releasing factor analog and (or) thyrotropin releasing hormone. J Anim Sci, 69: 1133-1139. [ Links ]
Spicer, L.J. and Echternkamp, S.E. 1995. The ovarian insulin and insulin-like growth factor system with an emphasis on domestic animals. Domest Anim Endocrinol, 12: 223-245. [ Links ]
Spicer, L.J., Chamberlain, C.S. and Maciel, S.M. 2002. Influence of gonadotropins on insulinand insulin-like growth factor-I (IGF-I)-induced steroid production by bovine granulosa cells. Domest Anim Endocrinol, 22: 237-254. [ Links ]
Strauss, J.F. and Barbieri, R.L. 2009. Yen and Jaffe's reproductive endocrinology series, physiology, pathophysiology and clinical management. W.B. Saunders Company, Limited. USA. 194 pp. http://www.yen%20and%20jaffe's%20reproductive%20goggle%20books.mht (06/12/2011). [ Links ]
Veldhuis, J.D. and Rodgers, R.J. 1987. Mechanisms subserving the steroidogenic synergism between follicle-stimulating hormone and insulinlike growth factor I (somatomedin C). Alterations in cellular sterol metabolism in swine granulosa cells. J Biol Chem, 262: 7658-7664. [ Links ]
Recibido:15-2-11
Aceptado: 13-4-11.