My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Archivos de Zootecnia
On-line version ISSN 1885-4494Print version ISSN 0004-0592
Arch. zootec. vol.63 n.242 Córdoba Jun. 2014
https://dx.doi.org/10.4321/S0004-05922014000200003
Nutrient utilization during incubation and juvenile growth of indigenous and exotic chicken in Nigeria
Utilización de nutrientes durante la incubación y crecimiento juvenil de pollos indígenas y exóticos en Nigeria
Obanla, L.O.; Oke, O.E.*; Onagbesan, O.M.; Williams, T.J.; Abioja, M.O.; Daramola, J.O. and Abiona, J.A.
Department of Animal Physiology. Federal University of Agriculture. Abeokuta. Nigeria
*emaoke7@yahoo.co.uk
SUMMARY
Nutrient utilization and early growth rate in three strains of chicken were investigated using 900 hatching eggs, 300 from each strain. The strains of chicken used were Nigerian indigenous chicken (NIC), ISA Brown (IB) and Nera Black strains (NB). Ten eggs per strain were randomly selected for breakout at embryonic day (ED) 7, 11, 15, and 18 of incubation to collect data on albumen weight in order to determine the embryonic albumen reduction rate during incubation and yolk weight to monitor its utilization. Blood samples were collected at hatch (day-old), weeks 1, 2, 3 and 4 post-hatch for triiodothyronine (T3), thyroxine (T4) and corticosterone level (CORT) determination. The chicks were randomly distributed into four rearing pens for a 28-day assessment of growth rate. The results showed that at day 18 of incubation (ED 18), weight of egg yolk and rate of yolk loss were similar among all the strains. Also, from ED 0 to 7 and11 to 15, albumen reduction rate in the eggs of IB was higher compared to NB and NIC, while it was lowest in NIC. From ED7 to 11, NIC showed highest reduction rate, followed by NB. Lowest reduction rate was shown in the eggs of IB. At day 7 and 28 of post-hatch growth, relative weight gain by the NIC was higher compared to NB and IB chicks. While IB and NB strains were similar at day 7, NB showed an intermediate relative weight gain at day 28. Strain did not significantly (p>0.05) affect body weight at all the ages. At day 14 and 21 of post-hatch growth, strain did not affect relative weight gain except at day 7 and 28. At day 7 and 28 relative weight gain by the NIC chicks was higher compared to NB and IB chicks.
While IB and NB were similar at day 7, NB showed an intermediate relative weight gain at day 28. Plasma CORT level did not change from day-old until day 28 in all strains. Similarly, CORT levels did not differ among strains at each age of determination. T3 concentration increased from day-old until day 7 post-hatch and leveled out throughout the ages of observation in all the strains. The weight differences of the embryos at ED18 and day-old-chicks at the hatching day suggest the influence of genetic differences and possibly that of inadequate incubation protocol at this stage.
Key words: Hormones.
RESUMEN
En tres líneas de pollos (Nigerian indigenous chicken, NIC; ISA Brown, IB y Nera Black, NB) se estudió la utilización de nutrientes durante la incubación y crecimiento juvenil, empleando 900 huevos (300 de cada línea). A los días embrionarios (ED) 7, 11, 15 y 18 de la incubación, diez huevos por línea fueron seleccionados al azar, para obtener datos sobre el peso del albumen y determinar su tasa de reducción embrionaria y peso de la yema. Se obtuvieron muestras de sangre con un día de edad (a la eclosión) y a las semanas 1, 2, 3 y 4 desde la eclosión para determinacion de triiodotironina (T3), tiroxina (T4) y corticosterona (CORT). Los pollos fueron distribuidos al azar en cuatro jaulas de cría para la evaluación del crecimiento durante 28 días. Los resultados demuestran que al día 18 de la incubación (ED18), el peso de la yema y la tasa de su consumo fueron similares en todas las líneas estudiadas. Además desde ED0 a ED7 y desde ED11 a ED15, la tasa de reducción del albumen en los huevos IB fue mayor que la de NB y NIC, siendo la de NIC la más baja. Desde el ED7 al ED11 NIC mostró la mayor velocidad de reducción seguida de NB, siendo la menor la de huevos IB. En los días 7 y 28 de crecimiento, la ganancia relativa de peso fue mayor en los pollos NIC, resultando similares entre sí los de IB y NB al día 7; NB demostró un crecimiento relativo intermedio al día 28. La línea no afectó significativamente al peso corporal en nuinguna etapa. En los días 14 y 21 de los pollos, la línea no afectó a la ganancia relativa de peso pero, a los 7 y 28 días de edad, la ganacia relativa de peso fue superior en los pollos NIC. IB y NB tuvieron incrementos relativos de peso similares al día 7 pero el de NB al día 28 fue intermedio. En ninguna línea el nivel plasmático de CORT varió desde el día 1 al 28, tampoco hubo diferencias entre líneas. La concentración de T3 aumentó desde el día 1 hasta el 7, estabilizándose después en todas las edades y cepas. Las diferencias de peso entre los embriones al ED18 y los pollos de un día, el día de la eclosión, sugieren la posibilidad de diferencias genéticas y posiblemente un inadecuado protocolo de incubación en esta etapa.
Palabras clave: Hormonas.
Introduction
The post-hatch performance of chicken is determined by genotype, environmental and feed-related factors (Bruggeman et al., 2006). Besides, when chickens are selected for various post-hatch characteristics, they may also differ in their embryonic developmental trajectories (Clum etal., 1995). Avian embryos develop and grow from nutrients stored in the egg over a wide range of time (Schmidt et al., 2003). Amounts and forms of nutrients deposited in the egg determine the success of embryo development and hatching of a healthy chick. Although, not derived from egg, oxygen and carbondioxide play a supportive role during incubation. However, adequate oxygen and carbon-dioxide concentrations in the surrounding air and its free passage through shell porosities are essential (Decuypere et al., 2001). Developmental limitations and embryo mortality are aggravated when any inadequacy in these conditions prevails.
A lot of research findings and reports have been published among which are yolk assimilation during embryonic development of chick (Jull and Heywang, 1930); effect of egg size on embryonic growth (Hazzan and Nordskog, 1971), Comparison of different strains of exotic chicken embryo physiology and chick juvenile growth (Tona et al., 2010), effect of high incubation temperature on layer chicken embryo (Tona et al., 2012) and all the likes. These researches have been able to examine various strains of exotic chickens. However, there is a dearth of information on the optimum incubation condition, hormonal profile and growth trajectory of the indigenous chicken in Nigeria.
Layer chickens have been intensively selected for egg production (Sato et al., 2009). Extensive experiments have investigated the physiological difference between chicken types, but these studies are usually limited in the NIC. Although, studies on embryonic development of exotic strains of chickens have been conducted in the temperate regions, none of the Nigerian studies has reported the embryonic development of the NIC strains vis-avis the post-hatch juvenile growth. The question to be asked is whether the currently used incubation protocols used for exotic strains are suitable for the NIC. Thus, a comparison of embryonic nutrient utilization and post-hatch performance among these strains of chickens might be 9a pointer focusing to areas of developing NIC for high performance and developing suitable incubation requirements for different strains. This study therefore aimed to compare some physiological parameters during embryonic development and post-hatch juvenile growth in the Nigerian indigenous normal feathered chicken with two commercial strains of layer chickens (NB and IB) of high performance in order to establish if the incubation conditions presently used for the indigenous chickens are adequate and also to establish if the exotic laying chickens have similar growth trajectories with the NIC.
Materials and methods
EXPERIMENTAL SITE
The experiment was conducted at the Research Farm of the Federal University of Agriculture Abeokuta (FUNAAB), Abeokuta, Nigeria. The climate is humid with a mean annual rainfall of 1037 mm. The annual mean temperature and humidity are 34 oC and 82 % respectively level (Amujoyegbe et al., 2008).
EGG COLLECTION AND INCUBATION
900 hatching eggs (300 per strain) from 2 strains of exotic chickens (IB and NB) were collected from commercial farms in Nigeria. Hatching eggs from the NIC were obtained from Abeokuta, Nigeria. Age of the birds was not put into consideration. It was ensured that the eggs were devoid of physical defects as these might interfere with embryo development and hatchability. The eggs were stored for 3 days in the cold room at the temperature of 18 oC prior to setting in the incubator. The incubating eggs from each strain were replicated twice at 150 eggs per replicate. The eggs were numbered for identification and set in the same incubator. The eggs were positioned in the setting crates with broad end facing up (Alabi et al., 2012) to ensure ease of gas exchange (CO2 and O2) between the eggs and the environment.
INCUBATION PROTOCOL
Single Stage Western® Incubator was used for setting of the eggs at a temperature of 37.8 oC and 60 % relative humidity (R.H.) with oxygen concentration of 20 %. At day 19 R.H. was increased to 70 %. Towards day 21 when chicks were likely to hatch, R.H. was reduced to 60 %. This was to allow chicks to dry off before being taken out of the hatcher. Turning of eggs was automatically done by the incubator at an angle of 90o hourly until day 18. Turning ensures even distribution of nutrients and prevents adherent of embryo to egg shell.
INCUBATION PHASE PARAMETERS
Ten eggs per strain were randomly selected for breakout at ED7, 11, 15, and 18 of incubation to collect data on: yolk weight (Yw) and albumen weight (Aw) to determine the albumen reduction rate (RAR) during incubation.
RAR= (Initial Aw-Final Aw)100/Initial Aw
POST-HATCH PHASE
The newly hatched chicks were removed from the hatcher and identified with their corresponding eggs. The chicks were weighed using a Mettler top-loading weighing balance.
At hatch, 60 Pullet chicks (20 per replicate) from each of NB and IB, and 60 from the NIC chicks (20 per replicate) were tagged for identification. They were brooded for four (4) weeks in an open sided brooder house supplied with heat depending on weather condition. The amount of heat supplied was based on the response of the chicks to the heat. The brooder house was partitioned and the chicks from each strain were assigned pens.
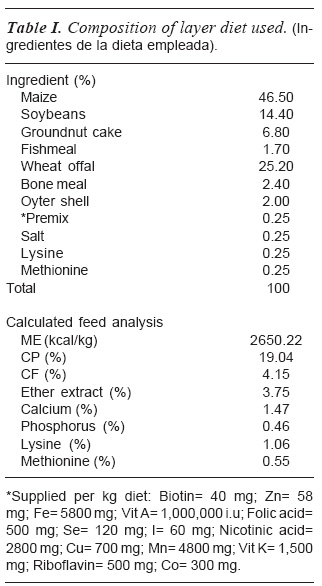
FEEDING MANAGEMENT AND WEIGHT GAIN DETERMINATION
The chicks were fed ad libitum with mash (Crude Protein of 19 % and Metabo-lisable Energy of2,650 per kilogramme) (as shown in table I) and clean water. Data were collected at day 7, 14, 21 and 28 for weight gain of individual birds using a Mettler top-loading weighing scale.
BLOOD SAMPLES COLLECTION
At day 0, 7, 14, 21, and 28 of post-hatch growth, 10 chicks per strain were selected and blood samples were aspirated from wing vein or jugular vein using 2 mL syringe. The blood samples were put in to heparinized tubes to avoid blood clotting. The blood samples were centrifuged at 3000 revolution per minute for 10 minutes to separate blood plasma. The plasma samples were labeled for identification and later frozen at the temperature of -20 oC until ready for analysis to determine the levels of corticosterone, tri-iodothyronine (T3) and thyroxine (T4) using radio immunoassay (RIA) technique as described by Darras et al. (1992). The analysis was carried out in K.U. Laboratory of Livestock Genetics, Immunology and Physiology, Leuven, Belgium.
STATISTICAL DESIGN AND ANALYSIS
The data were analyzed using a completely randomized design. The model is shown below:

All the data collected were subjected to Analysis of Variance using SAS (1999) statistical package while significant means were compared using Duncan's Multiple Range Test.
Results
COMPARISON OF EGG YOLK WEIGHT (G), % YOLK WEIGHT AND % YOLK LOSS DURING
INCUBATION
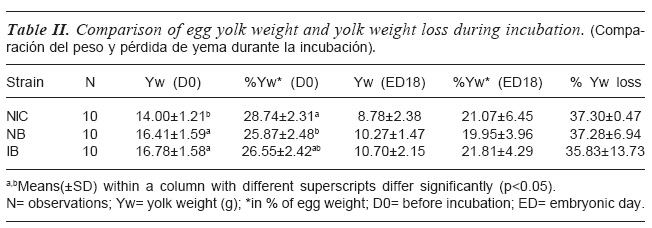
Yolk weight was compared among the strains of chicken at day 0 (pre- incubation) and day18 of incubation, as shown in table I. The table II shows that strain had significant effect (p<0.05) on egg yolk weight and percentage yolk weight prior to incubation (i.e. day 0). Prior to incubation egg yolk weights were similar between IB and NB chicken strains but greater in values than the egg yolk of the local chicken strain. The Local and IBhad similar percentage yolk weight, while NB showed the lowest weight which was similar with IB. At day 18 of incubation, egg yolk weights, percentage yolk weights, and percentage yolk loss were similar among all the strains.
COMPARISON OF ALBUMEN WEIGHT AND RELATIVE ALBUMEN REDUCTION DURING INCUBATION (%)
Table III shows the albumen weight and relative albumen reduction (%) in the incubating egg of the 3 strains of chicken. It shows significant effect of chicken strains on the albumen reduction rate in the eggs at different incubation durations. From embryonic day (ED) 0 to 7 and11 to 15, reduction rate in the eggs of IB strain was higher compared to NB and NIC, while NIC showed the lowest. From ED7 to 11 NIC showed highest reduction rate, followed by NB. Lowest reduction rate was shown in the egg of IB.
COMPARISON OF POST HATCH JUVENILE BODY WEIGHT (G) AND RELATIVE WEIGHT GAIN (%)
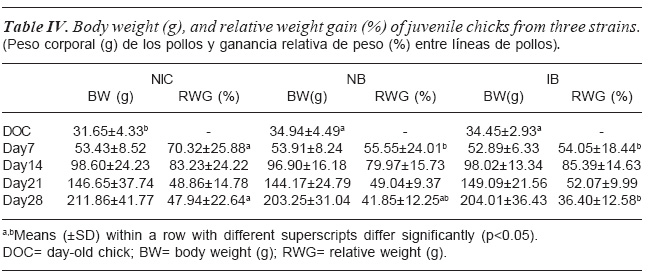
The post hatch juvenile chicks body weight and relative weight gain as affected by strain is shown in table IV. Strain did not significantly (p>0.05) affect body weight at all the ages. At day 14 and 21 strain did not affect relative weight gain except at day 7 and 28. At day 7 and 28 relative weight gain by the NIC chicks was higher compared to NB and IB chicks. While IB and NB were similar at day 7, NB showed an intermediate relative weight gain at day 28.
COMPARISON CORTICOSTERONE (CORT), TRIIODOTHYRONINE (T3) AND THYROXINE
(T4) concentrations (ng/mL) at hatch and post-hatch juvenile growth among three strains of chicken.
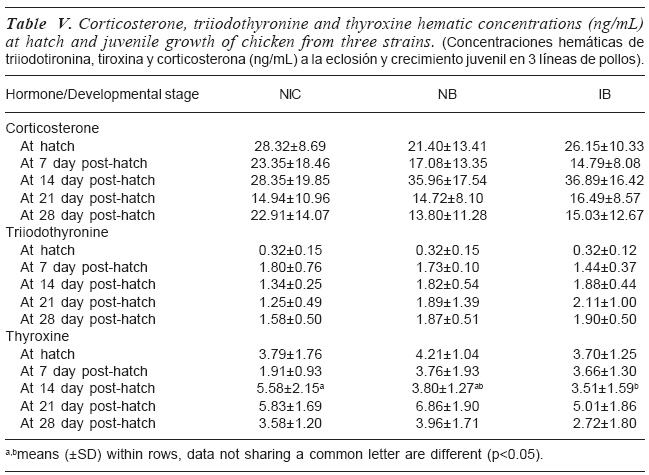
The concentrations of corticosterone (CORT), T3 and T4 at hatch and during juvenile development of the NIC, NB, and IB are shown in table V. Plasma CORT level did not change from day-old until day 28 in all strains. Similarly, CORT levels did not differ among strains at each age of determination. T3 concentration increased from day-old until day 7 post-hatch and leveled out throughout the ages of observation in all the strains. Similarly, T3 levels did not differ among strains at each age of determination. At each age of observation, strain did not significantly (p>0.05) affect T4 concentration except at day 14 in which Local strain had significantly (p<0.05) higher T4 compared to that of the NB and IB. NB showed similar value as the IB.
Discussion
The rate of reduction of albumen during incubation was not the same in the strains of chicken in this study. The reason for this may be attributed to genetic differences and evaporation loss, although each of the strains had similar embryo weights until day 15 of incubation. The similarity in the percentage yolk losses among the strains during embryonic development suggests that similar amount of yolk content were utilized by the strains. Finklera et al. (1998) indicated that differences in yolk seem not to result in greater tissue formation during embryonic development.
The superiority of the NIC chicken in post-juvenile relative weight gain over the other two strains at day (d)7 and 28 was seen except at d14 and 21 when they were similar. This could be attributed to genetic difference. Similar results were observed among chickens from different ecological zone by Adedokun and Sonaiya (2001) where weights of the eco-type chickens observed from different ecological zones varied and were similar at different growth trajectory during post-juvenile development. The superior body weights and relative body weights in the NIC at day 28 in this study reveals that the NIC has higher ability to catch up with the exotic strains and also overtake them despite the fact that the NIC was smaller than the exotic strains at hatch.
Corticosteroids are known to be elevated by corticotrophin-releasing hormone and stimulate thyroid metabolism in the chicken embryo, which is related to the hatching process (Decuypere et al., 1990). The production of glucocorticoids is increased by stress. Therefore, corticosterone can be used as a biomarker of stress. The similarity in corticosterone concentrations among the strains at each of the developmental stage in the present study indicates similarity in their ability to tolerate stress from the environment with little effect on performance. The finding is similar to the report of De Smit et al. (2005) where two lines of broilers had similar corticosterone levels. In contrast an ascites susceptible chicken which struggled to maintain metabolic rate showed a higher concentration of corticosterone.
The reason for higher plasma concentration level of T4 compared with T3 probably was due to the fact that elevated level of T4 concentration is a substrate for T3 production (Decuypere et al., 1990). This pattern conforms to the result of Tona et al. (2004). The similarity observed in the thyroid hormones (T3 and T4) levels among the strains except at day 14 of T4 is an indication that the strains of chicken had similar metabolic rate. This result is similar to De Smit etal. (2005) in which two lines of broiler maintained the same T3 levels, hence similar metabolic rate. Generally, plasma T3 levels of these strains at day 7 were higher than at hatch. It shows that it increased from hatch to day 7 and then leveled out. This means that the metabolic rate of each strain at hatch was lower than at day 7. In contrast, plasma T4 levels at these two stages of development were similar in the strains. This result is in contrast with the finding of De Smit et al. (2005) where T4 concentration level at day 7 was higher compared to the concentration at hatch at the same time in all the strains.
It could be concluded that the differences in albumen reduction rate during incubation indicated that the NIC embryo had higher conversion rate of albumen into body tissues than in the IB and NB strains at ED7 and 15. Moreover, the NIC chicks showed superiority in post-juvenile relative weight gain over the other two strains at day (d) 7 and 28, except at d14 and 21 when they were similar. This suggests that different strains may have different growth trajectory at different periods of post-juvenile development. This is in accordance with the findings of Tona etal. (2012) who observed different growth trajectories in different strains of broiler. There is however a dearth of information on the comparison of this parameter in the NIC and the exotic strains. Also, the three strains of chicken seemed to have similar tolerance to stress as reflected in their similar concentrations of plasma corticosterone. The Similarity in the blood plasma concentrations of thyroid hormones of the strains suggests that the NIC, NB and IB chicken have similar metabolic rates during post-hatch juvenile growth (0 to 28 days).
References
1. Adedokun, S.A. and Sonaiya, E.A. 2001. Comparison of the performance of Nigerian indigenous chickenfrom three agro-ecological zones, Livest Res Rural Dev, 13: 1-6. [ Links ]
2. Alabi, O.J.; Ng'ambi, J.W. and Norris, D. 2012. Effect of Egg Weight on Physical Egg Parameters and Hatchability of Indigenous Venda Chickens, Asian J Anim Vet Adv, 7: 166-172. [ Links ]
3. Amujoyegbe, B.J.; Bamire, A.S. and Elemo, K.O. 2008. Agronomic analysis of fertilizer effect on maize/cowpea intercrop in Ile-Ife and Abeokuta, South-western Nigeria. Asset Series A, 8: 62-72. [ Links ]
4. Bruggeman, V.; Tona, K.; Onagbesan, O. and Decuuypere, E. 2006. Hatching egg and chick quality. Biology breed of poultry. (Ed. P.M. Hocking). CAB International. UK. pp. 224-239. [ Links ]
5. Clum, N.J.; Mc Clearn, D.K. and Barbafo, G.F. 1995. Comparative embryonic development in chickens with different patterns of postnatal growth. Growth Develop Aging, 59: 129-138. [ Links ]
6. Darras, V.M.; Visser, T.J.; Berghman, L.R. and Kuhn, E.R. 1992. Ontogeny of type I and type III deiodinase activities in embryonic and posthatch chicken: relationship with changes in plasma triiodothyronine and growth hormone levels. Comp Biochem Physiol, 103A: 131-136. [ Links ]
7. De Smit, L.; Tona, K.; Brugeman, V.; Onagbesan, O.; Hassanszadeh, M.; Arckens, L. and Decuypere, E. 2005. Comparison of the three lines of broilers differing in ascites susceptibility or growth. Poultry Sci, 84: 1446-1452. [ Links ]
8. Decuypere, E.; Dewil, E. and Kühn, E.R. 1990. The hatching process and the role of hormones. In: Avian Incubation. S.G. Tullett (Ed.). Butterworth-Heinemann. London, UK. pp. 239-256. [ Links ]
9. Decuypere, E.; Tona, K.; Bruggeman, V. and Bamelis, F. 2001. The day-old chick: a crucial hinge between breeders and broilers. World Poultry Sci J, 57: 127-138. [ Links ]
10. Finklera, M.S.; Van Ormaná, J.B. and Sotherland, P.R. 1998. Experimental manipulation of egg quality in chickens: influence of albumen and yolk on the size and body composition of near-term embryos in a precocial bird. J Comp Physiol, B, 168: 17-24. [ Links ]
11. Hazzan, E.M. and Nordskog, A.W. 1971. Effect of egg size and heterosis on embryonic growth and hatching speed. Genetics, 67: 279-285. [ Links ]
12. Jull, M.A. and Heywang, B.W. 1930. Yolk assimilation during the embryonic development of the chick. Poultry Sci, 9: 393-404. [ Links ]
13. SAS Institute. 1999. SAS User's Guide: Statistics Inc. Cary, N.C. pp. 923. [ Links ]
14. Sato, M.; Tsuneyoshi, Y.; Sato, K. and Furuse, M. 2009. Comparison of 3-hydroxyacyl CoA dehydrogenase activity between broiler and layer chickens during embryonic development. Res J Bio Sci, 4: 585-587. [ Links ]
15. Schmidt, G.S.; Figueiredo, E.A.P.; Ledur, M.C. and Alves, H.J. 2003. Effect of selection for productive traits in broiler maternal lines on embryo development. Rev Bras Cienc Avic, 5: 125-129. [ Links ]
16. Tona, K.; Onagbesan, O.; De Ketelaere, B.; Decuypere, E. and Bruggeman, V. 2004. Effects of age of broiler breeders and egg storage on egg quality, hatchability, chick quality, chick weight, and chick post-hatch growth to forty-two days. J Appl Poultry Res, 13: 10-18. [ Links ]
17. Tona, K.; Onagbesan, O.M.; Kamers, B.; Everaert, N.; Bruggeman, V. and Decuypere, E. 2010. Comparison of Cobb and Ross strains in embryo physiology and chick juvenile growth. Poultry Sci, 89: 1677-1683. [ Links ]
18. Tona, K.; Kamers, B.; Evereart, N.; Gbearssor, M. and Decuypere, E. 2012. Effect of high incubation temperature on layer chicken embryo physiology. Proceeding of Association Mondiale des Sciences Avicoles. University de Lome. Togo. pp. 61-62. [ Links ]
Recibido: 9-10-12
Aceptado: 12-6-13