My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Archivos Españoles de Urología (Ed. impresa)
Print version ISSN 0004-0614
Arch. Esp. Urol. vol.61 n.9 Nov. 2008
MONOGRÁFICO: ENDOUROLOGÍA Y LÁSER
Treatment of the pyelocalyceal tumors with laser
Tratamiento de los tumores pielocaliciales con láser
Cesare Marco Scoffone, Cecilia Maria Cracco, Massimiliano Poggio, Marco Cossu, Roberto Mario Scarpa.
Department of Urology. San Luigi University Hospital. Orbassano. Turin. Italy.
SUMMARY
Transitional cell carcinoma of the upper urinary tract (UUT-TCCj is relatively uncommon, accounting for 2-5% of all urothelial tumors. Its incidence appears to be increasing as a result of progress in imaging, endoscopy, and improved survival from bladder cancer. Renal pelvis tumors represent 10% of all renal cancers. Pyelic neoplasms occur at a rate twice to four times the incidence of tumors in the ureter, where the common site is the distal tract (about 70%). One third of UUT-TCC are multifocal, and about 1% are simultaneous and bilateral.
The two alternative endoscopic approaches for lesions localized in the renal collecting system are the ureteroscopic retrograde one and the percutaneous anterograde one. The treatment choice is addressed by some criteria: lesion size, location, and multifocality. Small accessible lesions <l cm are preferably treated ureteroscopically, maintainig the integrity of the urinary tract.
The introduction of lasers represented a big step in the diagnosis and endoscopic treatment of upper urinary tract tumors. A successful laser treatment is defined by the careful selection of the patients affected by urinary tract lesions. Usually, only patients affected by low grade and papillary lesion should be treated endoscopically with laser. Patients with high grade and invasive lesions should rather be submitted to surgical procedure. Actually, the urologist has a wide choice in laser technology (Holmium laser, Thulium laser). For a correct and safe treatment of ureteral and pyelic lesions with lasers it is mandatory to respect some technical advises. First of all, an adequate access for a good vision of ureter and renal pelvis is imperative. In fact, the urologist should always work in safety, with an optimal control of the instrumentation. Then, it is important to define the laser type and its energy level. The development in laser technology (i.e. small and flexible laser fibers) allows also a radical, safe and minimally invasive treatment of urothelial lesions using flexible ureteroscopes. Of course it is mandatory to evaluate the grade and stage of the tumors by means of the ureteroscopic biopsies: invasive tumors must be treated by immediate nephroureterectomy while the endoscopic treatment should be reserved to those patients with a solitary kidney, renal failure, bilateral tumors, severe comorbities or affected by a solitary tumors with <15 mm in diameter and of low-grade/stage.
Keywords: Pyelocalyceal tumors. Ureteroscopy. Percutaneous surgery. Laser.
RESUMEN
El carcinoma de células transicionales del tracto urinario superior es relativamente raro, supone entre el 2-5% de todos los tumores uroteliales. Su incidencia parece estar aumentando como resultado de los progresos en técnicas de imagen, endoscopia y la mejoría de la supervivencia del carcinoma vesical. Los tumores de la pelvis renal representan el 10% de todos los cánceres renales. Las neoplasias piélicas tienen una incidencia de dos a cuatro veces superior a la de los tumores de uréter, de los que el sitio más común es el tracto distal (sobre el 70%). Un tercio de los carcinomas uroteliales del tracto urinario superior son multifocales, y el 1% simultáneos y bilaterales.
Los dos abordajes endoscópicos alternativos para las lesiones localizadas en el sistema colector del riñón son la ureteroscopia retrograda y la vía percutánea anterógrada. El tratamiento de elección depende de varios criterios: tamaño de la lesión, localización y multifocalidad. Las lesiones pequeñas < 1 cm accesibles se tratan preferentemente por ureteroscopia, manteniendo la integridad del tracto urinario.
La introducción de los láseres representó un gran paso del diagnóstico y tratamiento endoscópico de los tumores del tracto urinario superior. El éxito de un tratamiento láser en pacientes afectos de lesiones del tracto urinario viene definido por una selección cuidadosa. Generalmente, sólo los pacientes con lesiones de bajo grado y papilares deberían tratarse endoscópica mente con láser. Los pacientes con lesiones de alto grado o invasivas deberían mejor ser sometidos a intervención quirúrgica clásica. En realidad, el urólogo dispone de una amplia gama de tecnología láser para elegir (láser de Holmio, de Tulio). Para un tratamiento correcto y seguro de las lesiones ureterales y piélicas con el láser es absolutamente necesario respetar algunos consejos técnicos. Antes de nada, es imperativo un acceso adecuado para obtener una buena visión de uréter y de la pelvis renal. De hecho, el urólogo siempre debería trabajar con seguridad, con un control óptimo de sus instrumentos. Por lo tanto, es importante definir el tipo de láser y el nivel de energía; el desarrollo de la tecnología láser (por ejemplo fibras de láser flexibles y pequeñas) permite también un tratamiento radical, seguro y mínimamente invasivo de los tumores uroteliales utilizando ureteroscopios flexibles. Por supuesto es obligatorio evaluar el grado y estadio de los tumores después de la biopsia por ureteroscopia: los tumores invasivos deben ser tratados inmediatamente mediante nefroureterectomía, mientras que el tratamiento endoscópico debería reservarse para aquéllos pacientes con riñón único, insuficiencia renal, tumores bilaterales, comorbilidad severa o afectos de tumores solitarios con menos de 15 mm de diámetro y bajo grado/estadio.
Palabras clave: Tumores pielocaliciales. Ureteroscopia. Cirugía percutánea. Láser.
Epidemiology
Transitional cell carcinoma of the upper urinary tract (UUT-TCC) is relatively uncommon, accounting for 2-5% of all urothelial tumors. Its incidence appears to be increasing as a result of progress in imaging, endoscopy, and improved survival from bladder cancer. Renal pelvis tumors represent 10% of all renal cancers, and up to 60% are invasive at the time of first diagnosis. This is in contrast with the bladder, where 90% of the malignant tumors are urothelial carcinomas, and the majority of them (about 75%) start as superficial diseases (pTa, pTis, pT1). Pyelic neoplasms occur at a rate twice to four times the incidence of tumors in the ureter (1, 2), where the common site is the distal tract (about 70%) (3). One third of UUT-TCC are multifocal, and about 1% are simultaneous and bilateral.
Among patients with bladder cancer the rate of UUT-TCC ranges from 2% to 4%, whereas the incidence of bladder cancer in patients affected by UUT-TCC is 30-75% (1).
The peak of incidence of UUT-TCC is in the sixth and seventh decades of life. Among Caucasians the incidence is twice that occurring among African Americans.
An earlier detection of UUT-TCC could lead to a significantly better outcome for the patients, being 80% the 5-year survival rates for Ta tumors, 15-30% for T3 tumors. This is not so straightforward: in fact, in a study managed in the UK, the times to diagnosis of renal and ureteric TCC compare unfavourably with all other urological cancers, with a median of 48 days for UUT-TCC, compared to the 27 days for bladder TCC (4). This delay might be related to the relative difficulty in identifying small UUT lesions using the various combinations of the currently available endoscopic, imaging and laboratory investigations.
Diagnosis
Gross or microscopic hematuria is the most common presenting symptom of UUT-TCC (70% - 90% of patients). Flank pain, or a palpable mass, occur much less frequently (30% of patients). Symptoms like weight loss, anorexia, and bone pain are rarely present, unless the disease is advanced (1,5).
The tumor is found incidentally on an imaging study in 10% to 15% of patients. Historically, intravenous urography (IVU) has been used to evaluate the upper urinary tract, detecting a filling defect in 50% to 70% of cases (6). Nowadays, the preferred diagnostic tool for the upper urinary tract is CT scan with a delayed "urographic" phase, allowing the evaluation of both renal units and a better characterization of the lesions. Many benign disease processes may mimick a filling defect, including radiolucent stones, blood clots, fibroepithelial polyps, sloughed renal papillae, inverted papillomas, nephrogenic adenomas, subepithelial hematomas, fungal ball, extrinsic vascular compression, pyelitis or ureteritis cystica, tuberculosis, endometriosis, and amyloidosis (7). CT scan is accurate in distinguishing radiolucent stones (80-250 Hounsfield units [HU)) from soft tissue masses (10-70 HU). CT also provides 90% sensitive staging information. Unfortunately, it cannot differentiate Ta-T1 from T2 lesions, and has a 59% false-negative rate for the detection of invasiveness (8).
Standard evaluation of a UUT filling defect seen on IVU generally includes voided urine cytology, cystoscopy with retrograde pyelography, selective upper tract urine cytology and ureteropyeloscopy, with biopsy of suspect lesions. Retrograde pyelography is mainly performed to confirm the presence of a filling defect or for a better definition of the collecting system and ureteral anatomy. The sensitivity of urinary cytology correlates closely with the pathologic tumor grade. Selective uppertract cytology of high grade lesions, including carcinoma in situ, has a reported accuracy of detection of almost 80%, whereas for well-differentiated tumors it drops to 10% - 40% (9, 10).
Ureteropieloscopy in the diagnosis of upper urinary tract defects
Appropriate staging examinations should be carried out in a patient with a filling defect, in order to choose the most fitting surgical procedure. Staging mistakes are rather common, because contrast imaging cannot evaluate wall infiltration by UUT-TCC, which are already invasive or high grade at presentation in 30-55% of cases (11- 13). Ureteroscopy to evaluate an upper tract filling defect can greatly enhance diagnostic accuracy. In a retrospective study, ureteroscopy was demonstrated to have a diagnostic accuracy of 86% for renal pelvis tumors, and of 90% for ureteral tumors, compared to the accuracy of other standard approaches (respectively 55% and 52%) (14). Thanks to the advances in the design, optics, size, and deflectability of flexible ureteroscopes, the ureteropyeloscopy diagnostic accuracy is nowadays further increased. Bagley reported 25/62 (40%) UTT-TCC diagnosis after flexible ureteroscopy in patients with upper urinary tract filling defect; the recurrent sites were the proximal ureter and the renal pelvis (15). In addition to visualizing the upper tract, ureteroscopy offers the opportunity to biopsy any lesion encountered, allowing pathologic examination. For TCC, tumor grade and, only in some cases, tumor stage can be determined. Ureteroscopic biopsy should become a routine procedure, to help identifying the adequate candidates for a conservative or a radical procedure.
"Urinary tract grand tour". First of all, when a lesion of the upper urinary tract is suspected, it is mandatory to perform an endoscopic evaluation of urethra and bladder to diagnose possible urothelial tumors. Then, the procedure continues with the introduction of a semirigid or flexible ureteroscope into the ureteral orifice, performing a complete and detailed inspection of the entire circumferential wall of the distal and proximal ureter. It is important to perform these procedures under direct vision and, if it is possible, without a guide wire, to avoid ureteral and pelvis mucosa trauma mimicking a lesion. When the renal pelvis is reached, urine aspiration for selective cytology should be obtained; only after this step contrast medium is injected into the entire collecting system, ensuring a compete inspection. Endoscopic exploration includes all calyces, starting from the upper pole and proceeding to the medium calyces and lower pole.

When a lesion is found, first of all urine is taken at this level, then normal saline is used for local washings (16, 17). Biopsy of the lesion is performed with a flat-wire basket or 3 Fr biopsy forceps, followed by another saline wash and aspiration. Most biopsy forceps for flexible ureteroscopes have a limited cup size, yielding only minute samples that may be sufficient for pathologic diagnosis. Some authors reported the use of high resolution endoluminal ultrasound, to further evaluate features and depth of involvement of the renal pelvis wall and periureteral tissues. Guarnizo et al evidentiated the importance of taking multiple biopsies, and of obtaining deep specimens for the evaluation of the lamina propria (67,5%), using 11,5 Fr ureteroresectoscope (18).
Nephron-sparing surgery in UUT-TCC
The traditional treatment approach for UUT-TCC has just been for a long time nephroureterectomy with excision of bladder cuff, because of the aggressive nature of UUT-TCC, the likelihood of bladder recurrences, metachronous ipsilateral or even contralateral metastases. Renalsparing surgery was first proposed by Vest for ureteral tumors in 1945 (19), and by Ferris and Dent for renal pelvic malignancies in 1948 (20). The results obtained with segmental ureteral resection for proximal ureteral tumors, and surgical removal of tumors in the renal collecting system (50% and 45-65% recurrence rates) were not as favourable as open surgical resection of distal ureteral tumors with ureteral reimplantation. In a number of studies the high recurrence rates are even enhanced, when a portion of ureter is left intact, as reported by Mazeman (48% recurrence rates in patients submitted to simple nephrectomy, 12% after nephroureterectomy) (21, 22).

Nowadays, the development of improved optics, small calibre flexible endoscopes, ancillary instrumentation and applicable laser technology has given the urologist easier retrograde access to the entire upper urinary tract, leading to substantial changes in everyday practice. There is a trend towards a more conservative management of UUT-TCC, not only for those patients in whom extirpative surgery is contraindicated (solitary kidney, renal failure and comorbities), but also in patients with normal contralateral units.
The endoscopic approach in the treatment of UTT-TCC has been used since 1980: percutaneous tumor resection was proposed for renal calycopyelic tumors, while ureteroscopy has been effective for diagnosis and therapy of both ureteral and renal TCC.
The nephron-sparing procedures must be considered as a valid alternative to nephroureterectomy for various reasons.
First of all, in patient with a solitary kidney, it should be considered a basic procedure: infact the peak incidence for UUT-TCC is in the seventh decade when the 5 year survival rates of these patients on dialysis are very low (10-19%) (23).
Instead, nephroureterectomy could be considered an overtreatment in many patients with both renal units; in fact most of them have a low grade/stage disease, with good 5-year survival rates both after surgical treatment with distal ureterectomy (3, 24, 25) and the ureteroscopic and percutaneous approach (26, 27).
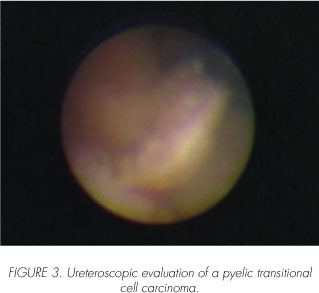
Tumor grade and stage have been identified as the most important determinants in predicting recurrence and survival for patients with UUT-TCC. Mufti and colleagues found the survival rate to be greater than 90% for patients with superficial well-differentiated tumors regard-less to treatment (total nephroureterectomy, or the more conservative endoscopic resection) (28). Charbit and associates found that 79% of grade 2 or 3 tumors invaded into or beyond the musc 1 e layer (29).
The rationale for endoscopic treatment in patients who have superficial bladder tumors or UUT-TCC is similar, since in both cases the entire tumor is potentially removable with this approach. However, important differences should be underlined. The wall thickness of ureter and renal collecting system is much thinner than that of the bladder, with an increased risk perforation, limiting a correct local pathologic staging. Additionally, endoscopy of the upper tract is more challenging, making therapy and follow-up technically difficult.
The endoscopic treatment in the renal collecting system lesions
The two alternative endoscopic approaches for lesions localized in the renal collecting system are the ureteroscopic retrograde one and the percutaneous anterograde one. The treatment choice is addressed by some criteria: lesion size, location, and multifocality. Small accessible lesions <l cm are preferably treated ureteroscopically, main-tainig the integrity of the urinary tract. These tumors often include lesions of ureter, renal pelvis, and upper calyces. Larger lesions, or lesions in less accessible calyces that cannot be resected or fulgurated adequately through a ureteroscope, can be managed more effectively percutaneously. Several endoscopic techniques are used. Even though there are many treatment options, each technique has limitations (27,30,32).
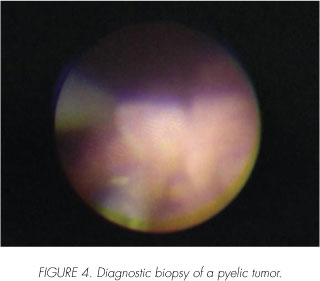
The laser in the diagnosis and treatment of UUT-TCC
The introduction of lasers represented a big step in the diagnosis and treatment of upper urinary tract tumors. In fact, delivering energy through a small and flexible fiber that can be used through all small, rigid and flexible endoscopes is a great advantage. Concerning the upper urinary tract tumors, electrosurgical devices have been replaced by lasers, which nowadays represent the best approach for conservative management.
The neodymium:yttrium-aluminum-garnet (Nd: YAG) and the Holmium YAG laser became the substitutes of the pulsed dye laser, and instrument of choice in stone fragmentation, and tissue cut and ablation.
In fact, tissue coagulation by electrosurgical devices, is necessarly followed by removal with graspers, avoiding ureteral lumen obstruction and subsequent difficult endoscopic accesses. The laser has overcome this problem by simultaneous coagulation and ablation of the tissue, thus clearing the small lumen to allow the advancement of the ureteroscope (33,34).
For a correct and safe treatment of ureteral and pyelic lesions with lasers it is mandatory to respect some technical advices. First of all, an adequate access for a good vision of ureter and renal pelvis is imperative. In fact, the urologist should always work in safety, with all optimal control of the instrumentation. Then, it is important to define the laser type and its energy level. In fact, the ureteral wall is very thin, and it is very easy to cause its injury or perforation. Using the right energy, the incidence of perforation is reduced, and guarantees less bleeding, and thus a good endoscopic vision.
Holmium YAG laser emits in the infrared region, at 2140 nm, with a pulse duration of 350 microsec. The Holmium YAG energy is absorbed efficiently in water, implying high safety margins in an aqueous environment, with tissue energy absorption, heat generation and vaporization. The emission of the laser beam is in a pulsed mode. Therefore, tissue penetration is less than 0,5 mm in depth and for this reason Holmium YAG laser fits very well for the treatment of urothelial lesions in the ureter and renal cavities, allowing an excellent hemostasis with minimal transmural thermal damage. It is transmitted efficiently by 150 to 600 micron low OH-silica fibers, passing through the thin working channel of the flexible ureteroscope. This laser seems to offer some advantages over other lasers used in urology. Perhaps, the most significant benefit is that the Holmium:YAG laser can potentially be used in almost all urological indication amenable to laser treatment including excision of soft tissue or lithotripsy, whereas all other wavelengths are restricted to one or few of these indication (35-37).
On the other hand, the continous Nd: YAG laser emits at 1060 nm, with less absorption and more penetration of energy. When setted at 20-30W, it is particularly effective for tissue coagulation at a considerable depth (56 mm), making it better suited for coagulative necrosis of large lesions, particularly in the renal pelvis. Still, it is less precise than the Holmium:YAG laser (36, 39).
In recent years, the Thulium: YAG laser (RevolixTM) has been introduced in the clinical practice. This laser operates at a wavelength of 2013 nm, and its surgical effect is entirely independent of vascularisation or tissue colour, since the laser energy is absorbed by interstitial water, which is ubiquitous in all tissues. Hemostasis is comparable to that of the Holmium:YAG laser, with the advantage of a continuous wave laser beam. This allows even more precise incision, combined with tissue vaporization. There are no reports in the literature describing UUT-TCC treatment by Thulium: YAG laser. In our experience we use the Thulium:YAG for the treatment of UUT-TCC: in fact, thanks to the opaqueness of the irrigant fluid, the thin urothelial wall in the ureter and renal pelvis is protected from the laser radiation. A successful laser treatment is defined by the careful selection of the patients affected by urinary tract lesions. Usually, only patients affected by a low grade and papillary lesion should be treated endoscopically with laser. Patients with high grade and invasive lesions should rather be submitted to surgical procedure (39).
Ureteroscopic treatment
Most UUT-TCC are endoscopically diagnosed and contextually treated. The best therapeutic strategy is planned after an accurate definition of lesion size, aspect and location. If the tumor is located in a favourable site such as the renal pelvis or upper calyx, the semirigid ureteroscope is the first choice. In fact its large working channel allows the use of a laser fiber with an adequate diameter. Instead, flexible ureteroscopes are used for the other sites, not easily accessible with the rigid endoscopes (i.e. medium and lower calyx). Gravity irrigation with normal saline is always performed, except when using the monopolar ureteroresector, implying the use of glycine. A ureteral access sheath should be inserted when possible, in order to avoid mucosal damage, safe multiple introductions of instruments and scopes, low-pressure continuous flow irrigation for excellent-visualization, removal of large specimens. Minimizing high-pressure irrigation during these procedures is recommended; high intra-renal pressures could promote pyelovenous or pyelolymphatic absorption/migration of malignant cells. A standard ureteral resectoscope loop is used for distal ureter lesions (11,5-13 Fr), taking care to avoid perforation of the very thin ureteral wall. In any case, this technique is characterized by bleeding, that may impair a good vision. Lesions localized in the proximal ureter and in the renal pelvis are difficult to resect, due to the rigid design of the ureteroscope, and are preferentially treated by laser fiber. Another approach, causing substantially less bleeding, is to perform cold-cup biopsies of the lesion using biopsy forceps, grasping devices, or a stone basket followed by laser fulguration. For larger tumors (> l cm), the Nd:YAG laser can be used to coagulate the bulk of the tumor, followed by ablation with the Holmium:YAG laser or Thulium:YAG laser. The Nd:YAG laser set at 30 W has been used with excellent results. The Nd:YAG laser has a depth of penetration of 5-6 mm, and is predominately used for deep tissue ablation and coagulation. Sometimes, when the tissue is burned, it becomes hard to evaluate the depth of fulguration and the amount of remaining tissue. For a safe use, it is important to remove recurrently the treated tumor, allowing an adequate exposure and treatment of progressively deeper layers. For an optimal performance, the Nd:YAG laser fiber should never be in contact with the tumor, avoiding the tip's charring. In two different studies Carson and Schilling reported treatment of ureteral TCC with the Nd YAG laser without recurrences at the follow up (40, 41).
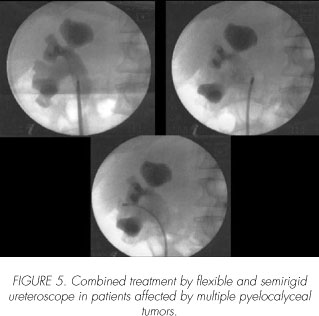
The Holmium and Thulium:YAG laser have a tissue penetration depth of 0.4 mm. They a superficial tissue ablation in the thin-walled ureter and renal collecting system, and are able to coagulate and control hemostasis. In contrast to the Nd:YAG laser, the Holmium:YAG laser fiber should be in contact with, or very close to the tissue to be ablated. The treatment with Thulium laser should be performed with the fiber either in direct contact with the lesion (10 W energy), or distant, using 15-20W energy, resulting in tissue vaporization (41, 42).
The currently available flexible ureteroscopes can accommodate laser fibers with ease; however, the placement of instruments or fibers through the working channel of a flexible ureteroscope significantly limits the amount of its active and passive deflection. Sometimes a tumor previously seen in a middle or lower calyx cannot be seen and reached any more when the laser fiber is passed. In such instances, it may be helpful to access the calyx before passing the fiber. Alternatively, a substantial amount of laser fiber can be advanced beyond the ureteroscope and directed into the calyx. The fiber acts as a guidewire, and allows the ureteroscope to be advanced over the excess fiber and into the calyx.
Percutaneous treatment
Ureteroscopic treatment of UUT-TCC is not considered an adequate therapy for patients with high-grade or invasive lesions. The increased rate of post operative residual tumors and the recurrence of tumors larger than 1.5 cm suggest that these lesions could be better managed by a percutaneous approach. The main advantages are the direct access into the desired calyx, even in case of anatomy abnormalities, a better visualization of the renal pelvis, low intrapelvic pressures, easier passage of a variety of accessories when large sheaths are used. The main disadvantage is the violation of urothelial integrity, with reported tumor seeding. The bulk of the tumor is removed by a cutting loop electrode or biopsy forceps, then the Nd:YAG laser, the Holmium and Thulium:YAG laser are used to refine resection, ablation and hemostasis (27, 43-47).
Results
Many series of patients affected by UTT-TCC and treated by means of laser ablation are reported, even though numbers and time of follow-up are usually small and the results very different. The recurrence rate reported ranges from 17 to 70%. Yamada et al. reported ureteroscopic treatment of five patients, with no recurrences with a mean 10.4 months follow up. Elliot et al. treated with Nd:YAG lasers two patients affected by low grade UTT-TCC, without recurrences at follow up (26). Chen and Bagley reported a series of patients affected by low stage disease, treated with a combination of Nd:YAG and Holmium:YAG: the recurrence rate was 64%. Jarret et. al stratified their 34 patients by tumor grade at diagnosis, and demonstrated that after percutaneous resection recurrence rates were 18, 33 and 50%, and death rates were 0,0 and 40% for grade 1, 2 and 3 TCC, respectively (47). A recent report of Traxer and co-workers (49) compares large series (97 patients) of patients affected by UUT-TCC, treated respectively with open nephroureterectomy and the endoscopic approach (ureteroscopic and percutaneous laser ablation, when possible). The 5-year disease-specific survival rate was 81.9% for low grade tumors and 47.3% for high grade tumors. In patients with low grade tumors (46/97), the 5-year disease specific survival rate after nephroureterectomy, ureteroscopy and percutaneous endoscopy was 84%, 80.7% and 80%, respectively; the corresponding 5-year tumor-free survival rates after endoscopy were 75.3%, 71 .5% and 72%. Another retrospective study (27) analyzed 24 patients submitted to percutaneous treatment of renal pelvis and calyceal tumors from 1989 to 2005 (18 treated by Nd and Ho: YAG laser); 33.3% of patients experienced local recurrence, while 20.8% underwent nephroureterectomy. The 5-year disease-specific and tumor-free survival rates were 79.5% and 68% respectively. In a series of 20 patients reported by Schmeller and Hofstetter (50) 4 patients were affected by high grade TCC, and the endoscopic approach was not enough to completely ablate the lesions. In our 6 years' experience, 71 patients affected by UUT-TCC (66% pyelocalyceal, 34% in the ureter) were submitted to diagnostic ureteroscopy with biopsy and laserization of the lesions. 52% (37/71) underwent to radical surgery (31 nephroureterectomy, 1 bilateral nephroureterectomy+ cistectomy in one session, 5 cistectomy+distal ureterectomy) for high grade and stage. 34 patients (48%) were treated only with endoscopic procedure: six of these have solitary kidney and the endoscopic treatment was the first choice even if the high grade or stage. 56% of these patients are disease-free (median follow-up: 41 months). Recurrent disease was developed in 41% of the patients treated conservatively, including all of those with a solitary kidney (4 patients submitted to other ureteroscopic treatments and 2 to partial nephrectomy). 3/34 patients (8%) were submitted to radical nephroureterectomy for high grade recurrent disease, while 4 (11%) had low grade recurrent disease and were submitted again to endoscopy (51).
Conclusions
Thanks to the evolution of endoscopic instrumentation, a primary conservative management of pyelocalyceal tumors becomes possible. In particular, the development in laser technology (i.e. small and flexible laser fibers), allows also a radical, safe and minimally invasive treatment of urothelial lesions using flexible ureteroscopes. Of course it is mandatory to evaluated the grade and stage of the tumors after the ureteroscopic biopsy: invasive tumors must be treated by immediate nephroureterectomy while the endoscopic treatment should be reserved to those patients with a solitary kidney, renal failure, bilateral tumors, severe comorbities or affected by solitary tumors with <15 mm of in diameter and of low-grade/stage.
References and recomended readings (*of special interest, **of outstanding interest)
*1. MESSING, E.M.; CATALONA, W.: "Urothelial tumors of the urinary tracty". Walsh, P.C.; Retik, A.B.; Vaughan, E.D. Jr. y cols., editors. Verduci Editore. Campbell's Urology. Vol. 5 7th Edition p.2411, 1999. [ Links ]
2. TAWFIEK, E.R.; BAGLEY, D.H.: "Upper-tract transitional cell carcinoma". Urology, 50: 321, 1997. [ Links ]
3. BABAIAN, R.J.; JOHNSON, D.E.: "Primary carcinoma of the ureter". J. Urol., 123: 357, 1980. [ Links ]
4. BAUS (Britsh Association of Urological Surgeons). Cancer Registry. www.baus.org. U.K. 2005. [ Links ]
5. GEERDSEN, J.: "Tumours of the renal pelvis and ureter. Symptomatology, diagnosis, treatment and prognosis". Scand. J. Urol. Nephrol., 13: 287, 1979. [ Links ]
6. FEIN, A.B.; McCLENNAN, B.L.: "Solitary filling defects of the ureter". Semin. Roentgenol., 21: 201, 1986. [ Links ]
7. MALEK, R.S.; AGUILO, J.J.; HATTERY, R.R.: "Radiolucent filling defects of the renal pelvis: Classification and report of unusual cases". J. Urol., 114: 508, 1975. [ Links ]
8. LANTZ, E.J.; HATTERY, R.R.: "Diagnostic imaging of urothelial cancer". Urol. Clin. North Am., 11: 567, 1984. [ Links ]
9. ZINCKE, H.; AGUILO, J.J.; FARROW, G.M. y cols.: "Significance of urinary cytology in the early detection of transitional cell cancer of the upper urinary tract". J. Urol., 116: 781, 1976. [ Links ]
10. GRACE, D.A.; TAYLOR, W.N.; TAYLOR, J.N. y cols.: "Carcinoma of the renal pelvis: A 15-year review". J. Urol., 98: 566, 1967. [ Links ]
11. OLGAC, S.; MAZUMDAR, M.; DALBAGNI, G. y cols.: "Urothelial carcinoma of the renal pelvis: A clinicopathologic study of 130 cases". Am. J. Surg. Pathol., 28: 1545, 2004. [ Links ]
*12. IBORRA, I.; SOLSONA, E.; CASANOVA, J. y cols.: "Conservative elective treatment of upper urinary tract tumors: A multivariate analysis of prognostic factors for recurrence and progression". J, Urol., 169: 82, 2003. [ Links ]
13. SCOLIERI, M.J.; PAIK, M.L.; BROWN, S.L. y cols.: "Limitations of computed tomography in the preoperative staging of upper tract urothelial carcinoma". Urology, 56: 930, 2000. [ Links ]
*14. BLUTE, M.L.; SEGURA, J.W.; PATTERSON, D.E. y cols.: "Impact of endourology on diagnosis and management of upper urinary tract urothelial cancer". J. Urol., 141: 1298, 1989. [ Links ]
*15. BAGLEY, D.H.; HUFFMAN, J.L.; LYON, E.S.: "Flexible ureteropyeloscopy: Diagnosis and treatment in the upper urinary tract". J. Urol., 138: 280, 1987. [ Links ]
16. LIATSIKOS, E.N.; DINLENC, C.Z.; KAPOOR, R. y cols.: "Transitional-cell carcinoma of the renal pelvis: Ureteroscopic and percutaneous approach". J. Endourol., 15: 377, 2001. [ Links ]
17. MURPHY, D.M.; ZINCKE, H.; FURLOW, W.L.: "Primary grade 1 transitional cell carcinoma of the renal pelvis and ureter". J. Urol., 123: 629, 1980. [ Links ]
18. GUARNIZO, E.; PAVLOVICH, C.P.; SEIBA, M. y cols.: "Ureteroscopic biopsy of upper tract urothelial carcinoma: Improved diagnostic accuracy and histopathological considerations using a multi-biopsy approach". J. Urol., 163: 52, 2000. [ Links ]
19. VEST, S.A.: "Conservative surgery in certain benign tumors of the ureter". J. Urol., 53: 97, 1945. [ Links ]
20. FERRIS, D.O.; DENT, R.V.: "Epithelioma of the pelvis of a solitary kidney treated by electrocoagulation". J. Urol., 59: 577, 1948. [ Links ]
21. MAZEMAN, E.: "Tumours of the upper urinary tract calyces, renal pelvis and ureter". Eur. Urol., 2: 120, 1976. [ Links ]
22. ZIEGELBAUM, M.; NOVICK, A.C.; STREEM, S.B. y cols.: "Conservative surgery for transitional cell carcinoma of the renal pelvis". J. Urol., 138: 1146, 1987. [ Links ]
23. National Kidney and Urological Disease Information Clearinghouse, National Institute of Diabetes and Digestive and Kidney Disease (NIDDK). National Institutes of Health, NIH Publication 04-3895, http://kidney-niddk.nih.gov. 2004. [ Links ]
24. ZINCKE, H.; NEVES, R.J.: "Feasibility of conservative surgery for transitional cell cancer of the upper urinary tract". Urol. Clin. North Am., 11: 717, 1984. [ Links ]
25. GIANNARINI, G.; SCHUMACHER, M.C.; THALMANN, G.N. y cols.: "Elective management of transitional cell carcinoma of the distal ureter: Can kidneysparing surgery be advised?". BJU Int., 100: 264, 2007. [ Links ]
26. ELLIOTT, D.S.; BLUTE, M.L.; PATTERSON, D.E. y cols.: "Long-term follow-up of endoscopically treated upper urinary tract transitional cell carcinoma". Urology, 47: 819, 1996. [ Links ]
**27. ROUPRÉT, M.; TRAXER, O.; TLIGUI, M. y cols.: "Upper urinary tract transitional cell carcinoma: Recurrence rate after percutaneous endoscopic resection". Eur. Urol., 51: 709, 2007. [ Links ]
28. MUFTI, G.R.; GOVE, J.R.; BADENOCH, D.F. y cols.: "Transitional cell carcinoma of the renal pelvis and ureter". Br. J. Urol., 63: 135, 1989. [ Links ]
29. CHARBIT, L.; GENDREAU, M.C.; MEE, S. y cols.: "Tumors of the upper urinary tract: 10 years of experience". J. Urol., 146: 1243, 1991. [ Links ]
30. SODERDAHL, D.W.; FABRIZIO, M.D.; RAHMAN, N.U. y cols.: "Endoscopic treatment of upper tract transitional cell carcinoma". Urol. Oncol., 23: 114, 2005. [ Links ]
31. ROUPRET, M.; CUSSENOT, O.; CHARTIER-KAST-LER, E. y cols.: "Role of endoscopy in the management of upper urinary tract tumors". Prog. Urol., 16: 45. 537, 2006. [ Links ]
**32. TRAXER, O.: "Ureteroscopic management of patients with upper urinary tract transitional cell carcinoma". Eur. Urol., 6: 560, 2007. [ Links ]
**33. BAGLEY, D.H.: "Ureteroscopic laser treatment of upper urinary tract tumors". J. Clin. Laser Med. Surg., 16: 551998. [ Links ]
34. FLORATOS, D.L.; DE LA ROSETTE, J.J.: "Lasers in urology". BJU Int., 84: 204, 1999. [ Links ]
35. PHILLIPS, C.K.; LANDMAN, J.: "Lasers in the upper urinary tract for non-stone disease". World J. Urol., 25:249, 2007. [ Links ]
36. JOHNSON, D.E.; CROMEENS, D.M.; PRICE, R.E.: "Use of the holmium: YAG laser in urology". Laser Surg. Med., 12: 353, 1992. [ Links ]
37. LARIZGOITIA, I.; PONS, J.M.: "A systematic review of the clinical efficacy and effectiveness of the holmium: YAG laser in urology". BJU Int., 84: 1, 1999. [ Links ]
38. LAM, J.S.; GUPTA, M.: "Ureteroscopic management of upper tract transitional cell carcinoma". Urol. Clin. North Am., 31: 115, 2004. [ Links ]
**39. TEICHMANN, H.O.; HERRMANN, T.R.; BACH, T.: "Technical aspects of lasers in urology". World J. Urol., 25: 221, 2007. [ Links ]
40. KAUFMAN, R.P. Jr.; CARSON, C.C. 3rd.: "Ureteroscopic management of transitional cell carcinoma of the ureter using the neodymium: YAG laser". Laser Surg. Med., 13: 625, 1993. [ Links ]
41. TEICHMANN, H.O.; DUCZYNSKI, E.W.; HUBER, G.: "Conference on lasers and electro-optics". Technical Digest Serioes, vol. 7, 1988. [ Links ]
42. TEICHMANN, H.O.; DUCZYNSKI, E.W.; HUBER, G.: "17 J Ho-laser at 2 microns". Weber, H.; (ed) Proc SPIE, vol 1021. High Power Solid State Lasers, 1998. [ Links ]
43. GOEL, M.C.; MAHENDRA, V.; ROBERTS, J.G.: "Percutaneous management of renal pelvic urothe-lial tumors: Long-term follow-up". J. Urol., 169: 925, 2003. [ Links ]
44. CHEW, B.H.; PAUTLER, S.E.; DENSTEDT, J.D.: "Percutaneous management of upper-tract transitional cell carcinoma". J. Endourol., 19: 658, 2005. [ Links ]
45. JABBOUR, M.E.; DESGRANDCHAMPS, F.; CAZIN, S. y cols.: "Percutaneous management of grade II upper urinary tract transitional cell carcinoma: The long-term outcome". J. Urol., 163: 1105, 2000. [ Links ]
*46. CLARK, P.E.; STREEM, S.B.; GEISINGER, M.A.: "13-year experience with percutaneous management of upper tract transitional cell carcinoma". J. Urol., 161: 772, 1999. [ Links ]
**47. JARRETT, T.W.; SWEETSER, P.M.; WEISS, G.H.: "Percutaneous management of transitional cell carcinoma of the renal collecting system: 9-year experience". J. Urol., 154: 1629, 1995. [ Links ]
48. CHEN, G.L.; BAGLEY, D.H.: "Ureteroscopic management of upper tract transitional cell carcinoma in patients with normal contralateral kidneys". J. Urol., 164: 1173, 2000. [ Links ]
*49. ROUPRET, M.; HUPERTAN, V.; TRAXER, O. y cols.: "Comparison of open nephroureterectomy and ureteroscopic and percutaneous management of upper urinary tract transitional cell carcinoma". Urology, 67: 1181, 2006. [ Links ]
50. SCHMELLER, N.T.; HOFSTETTER, A.G.: "Laser treatment of ureteral tumors". J. Urol., 141: 840, 1989. [ Links ]
51. SCOFFONE, C.; COSSU, M.; PORPIGIA, F. y cols.: "Trattamento ureterospopico conservativo dei tumore dell' alta via escretrice: nostra esperienza". p.16. ATTISIU ROMA, 2008. [ Links ]
 Correspondence:
Correspondence:
Scoffone Cesare Marco
Department of Urology
San Luigi University Hospital
Regione Gonzole 10
Orbassano. Turin. (Italy)
scoof@libero.it














