Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Archivos Españoles de Urología (Ed. impresa)
versión impresa ISSN 0004-0614
Arch. Esp. Urol. vol.62 no.4 may. 2009
Response to adjuvant chemotherapy after radical cystectomy in patients with infiltrative bladder: Analysis of 397 cases
Respuesta a la quimioterapia adyuvante post-cistectomía en pacientes con tumor de vejiga infiltrante: Análisis de 397 casos
J. I. Monzo Gardiner, F. Herranz Amo, R. Cabello Benavente, R. Diez Cordero, I. López Diez, J. Tobares Jiménez, R. Molina Escudero, E. Paños Facundo and C. Hernández Fernández
Department of Urology. Univesity General Hospital Gregorio Marañon. Madrid. Spain.
SUMMARY
Objectives: To define the usefulness of adjuvant chemotherapy in patients with pT2, pN0, pT3-4, pN0 and pN+ disease.
Methods: Retrospective analysis of 397 patients with transitional bladder cancer who underwent radical cystectomy between 1986 and 2005. Adjuvant chemotherapy was administered to 40.2% of patients. Three cycles of adjuvant MVAC (methotrexate, vinblastine, adriamycin and cisplatin) were given.
Results: In patients with pT3, pN0 (p=0.04) and/or N+ stages (p=0.001), adjuvant chemotherapy significantly improved cancer-specific survival, which did not occur in pT2N0 (p=0.9) and pT4, pN0 (p=0.6) patients. In the univariate analysis, adjuvant chemotherapy was significantly associated with a lower cancer-specific survival rate (RR 1.452 95% CI: 1.028- 2.057 p= 0.03), while the multivariate analysis showed a trend (RR: 0.651 95% CI 0.398-1.065, p=0.08) towards a decrease in cancer-specific mortality.
Conclusions: Although adjuvant chemotherapy was not shown to improve survival in patients with pT0-2, pN0 and pT4, pN0 disease, it did increase survival in those with extravesical disease, pathological state T3, pN0 and/or pN+. Considering its tendency to improve cancer-specific survival, adjuvant chemotherapy may be considered as a "protective factor" (RR=0.651, p=0.08).
Key words: Bladder cancer. Cystectomy. Chemotherapy.
RESUMEN
Objetivo: Conocer la utilidad de la quimioterapia adyuvante en los pacientes con enfermedad pT2, pN0, pT3-4, pN0 y pN+.
Métodos: Análisis retrospectivo de 397 pacientes con cáncer transicional de vejiga tratados mediante cistectomía radical entre el año 1986 y 2005. Al 40,2% de los pacientes se les administró quimioterapia adyuvante. Se administraron 3 ciclos de MVAC adyuvante (metotrexate, vinblastina, adriamicina y cisplatino).
Resultados: En pacientes con estadio pT3, pN0 (p=0,04) y/o N+ (p=0,001), la quimioterapia adyuvante aumentó la supervivencia cáncer-específica de forma significativa, no siendo así en pacientes pT2N0 (p=0,9) y pT4, pN0 (p=0,6). En el análisis univariante la quimioterapia adyuvante se asoció de forma significativa con una menor supervivencia cáncer-específica (RR 1,452 IC 95%: 1,028- 2,057p= 0,03) En el análisis multivariante presentó una tendencia (RR: 0,651 IC 95% 0,398-1,065, p=0,08) a la disminución de la mortalidad cáncer-específica.
Conclusiones: La quimioterapia adyuvante no demostró mejorar la supervivencia en pacientes con estadio pT0-2, pN0 y pT4, pN0. En cambio, la aumentó en los pacientes con enfermedad extravesical, estadio pT3, pN0 y/o pN+. Debido a la tendencia de la quimioterapia adyuvante a mejorar la supervivencia cáncer específica podemos considerarla como "protectora" (RR=0,651, p=0,08).
Palabras clave: Cáncer vesical. Cistectomía. Quimioterapia.
Introduction
Bladder cancer is the seventh most common tumor worldwide, accounting for 3.2% of all malignancies. The highest incidence for both sexes is seen in Europe, North America and Australia (1,2). It is also the fourth most common cancer in men (7% of all tumors) and the eight cause of cancer death (3% of all tumors). It is not included among the most prevalent tumors in women (3).
The most common hystologic type is the urothelial transitional carcinoma, which represents more than 90% of cases in Europe, North America, and Australia (4).
One third of the 63,210 new cases of bladder cancer diagnosed in 2005 showed an invasion of the muscular propia, while another 15-30% of high-grade superficial tumors will progress to an infiltrating tumor, most of them within 5 years following diagnosis. Radical cystectomy with pelvic lymphadenectomy is the mainstay therapy for infiltrating bladder cancer (5).
Despite improvements in both technical procedures and postoperative care, the analysis of series both before and after 1986 - when cisplatin was introduced as part of chemotherapeutic regimens - only showed a modest improvement (6). The AEU clinical practice guidelines do not include any criteria for the use of chemotherapy in patients with muscle-invading/invasive disease, although they state that clear evidence of any benefit from adjuvant chemotherapy is still lacking (7).
Approximately 50% of patients with infiltrating urothelial bladder cancer die within 3-4 years following radical cystectomy, which seems to be due to the fact that half of the patients show unsuspected distant metastases (micrometastases) at the time of cystectomy. Cystectomy is deemed to be an inadequate approach for the curative treatment of patients with extravesical disease with or without lymph node involvement (pT3-4, pN0/pN+) (5,8-14).
Objetives
To define the usefulness of adjuvant chemotherapy in:
Patients with localized disease (pT2, pN0).
Patients with locally advanced disease (pT3-4, pN0).
Patients with lymph node involvement (pN+).
Material and method
This is a retrospective analysis of 397 patients with transitional bladder cancer treated with radical cystectomy between 1986 and 2005. A review of clinical records was undertaken between October 2006 and July 2007. A total of 64 (16%) patients were excluded due to the following reasons: 21 (5.3%) due to death occurred during the immediate postoperative period, 32 (8%) because of previous radical radiotherapy with curative intent, 6 (1.5%) due to neoadjuvant chemotherapy, and 5 (1.2%) patients from other autonomous communities who were followed up at their reference centers. Thus, 333 patients remained valid for evaluation in the study.
Indications for cystectomy did not change throughout the study period. Cystectomy was indicated in patients with infiltrating bladder carcinoma (cT2 or higher), endocopically uncontrollable superficial tumor (cT1 or lower), BCG-resistant carcinoma in situ (Tis), and either persistence or relapse of bladder tumor after radical radiotherapy.
All of the patients had undergone at least one transurethral resection that confirmed both the existence of a primary transitional bladder carcinoma and its histological grade. Since 2002 they were pathologically categorized according to the TNM 2002 classification of the UICC (6th ed.), which was also used to reclassify those patients treated before 2002. Patients in whom no tumor was found on the cystectomy sample were classified as pT0. If lymph node invasion was detected, they were grouped as pN+. Patients in whom a lymphadenectomy was not performed were classified as pNx.
Four risk groups were defined according to the pathological findings in both the bladder and the lymph nodes observed in the lymphadenectomy specimen: (a) Localized bladder disease with no lymph node involvement (pT0-2, pN0); (b) Extravesical disease with no lymph node involvement (pT3-4, pN0); (c) Disease with lymph node involvement (pT0-4, pN+); (d) No available data on lymph node involvement (pT0-4, pNx).
Patients with both pTa (2 cases) and pTis (4 cases) stages were included as pT1 in the classification.
The criteria for the administration of adjuvant chemotherapy changed over the study period. Initially, it was given to patients showing at least an infiltrated deep muscular layer (pT2b) or a lymph node involvement (pN+), although it is now only administered to patients in the stage pT3 or higher or pN+. Three cycles of MVAC (metotrexate, vinblastine, adriamycin and cisplatin) were administered, although cisplatin was later replaced by carboplatin.
Patients were followed at our outpatient clinic at month 3, and then every 6 months up to year 5. From this time onwards, they were further followed annually.
The Fisher's exact test and the chi-square test were used to assess the association between categorical variables. To evaluate the differences between continuous distribution variables, either the Student t test or the Mann-Whitney test were used.
The Kaplan-Meier method was used to evaluate survival, while the log rank test was applied to assess the differences between the various groups with their 95% confidence intervals.
A univariate analysis was undertaken, which was later adjusted by using a Cox proportional risk model (ENTER method).
Statistical significance was defined as a p < 0.05 level. All calculations were done by using the Spanish version of the SPSS statistical software 12.0.
Results
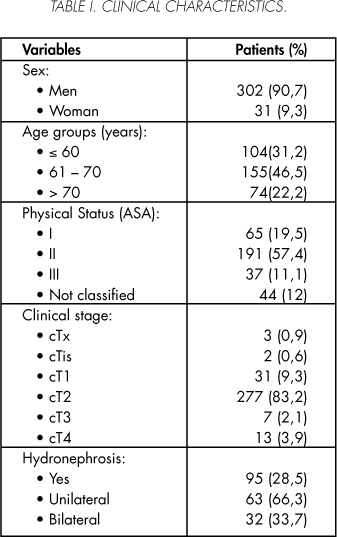
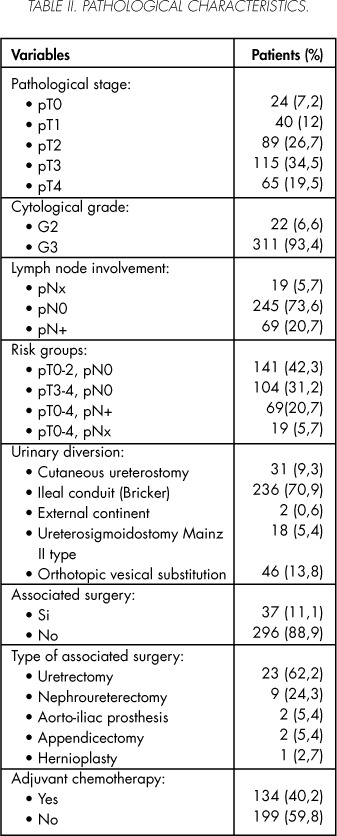
Of the 333 valid patients included in the study, 90.7% were males y 9.3% women. Their mean age was 63.4 ± 9 (31 - 89) with a median of 65 years. A cystectomy was performed by a staff member in 212 (63.6%) patients and by a resident assisted by a staff member in the 121 (36.4%) remaining patients. The patients' clinical characteristics are summarized in table I, while their pathological characteristics are described in Table II.
The mean follow-up period in the series was 52.6 ± 51 (2-221) months with a median of 31 months.
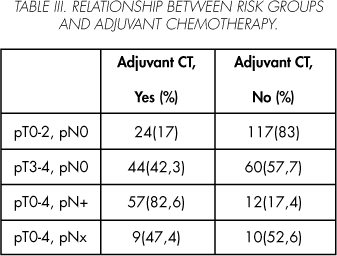
Adjuvant chemotherapy was administered to 40.2% of the study patients: 17% with localized disease, 58.3% with extravesical disease, and 82.6% with a lymph node involvement on the lymphadenectomy specimen (Table III).
The 20-year period covered by the series was divided into four 5-year intervals. During the last two 5-year period (1996-2000 y 2001-2005) a significant increase (p=0.02) in the age of operated patients was seen. No differences were observed either in sex (p=0.64) or pathological grade (p=0.67). The time elapsed between the TUR and the cystectomy was 2.17 ± 1.6 (0-14) months, with no significant differences being found between the different study periods. Significant differences were seen in the series (p=0.01) with respect to risk groups: an increase in extravesical disease was found (pT3-4, pN0) along with a decrease in intravesical disease (pT0-2, pN0), while lymph node involvement remained stable (pN(+)). A significant increase in the global use of adjuvant chemotherapy was seen (p=0.006), as well as a decrease in localized disease and an increase in extravesical disease (Table IV).
In 44 (13.2%) patients a second primary tumor in a another organ was diagnosed. The most commonly affected organs were the lung in 18 (5.4%) patients, and the gastrontestinal tract in 9 (2.7%); other 9 (2.7%) patients had a second urothelial tumor (8 cases in the upper urothelium and 1 case in the urethra).
At the end of the review, 143 (43%) patients had attained complete remission (disease-free), 21 (6%) had a local and/or distant relapse, and 169 (51%) had died. Among these latter patients, 130 (77%) died because of bladder cancer, 12 (7%) of another type of tumor, and 27 (16%) from other causes. The mean survival period in deceased patients according to the cause of death was: 21 (95% CI, 1824) months, with a median of 15 (95% CI, 13-17) for bladder cancer; 69 (95% CI, 33-105) months, with a median of 45 (95% CI, 30-60) months for another type of tumor and 48 (95% CI, 30-66) months, with a median of 24 (95% CI, 30-66) months in patients who died from other causes.
Global mean survival in the series was 103 (95% CI, 91-115) months, with a median of 61 (95% CI, 27-95) months. Five- and 10-year survival rates were 50% and 43%, respectively.
The mean cancer-specific survival was 129 (95% CI, 117-141) months. Five- and 10-year survivals rates were 64% and 54%, respectively.
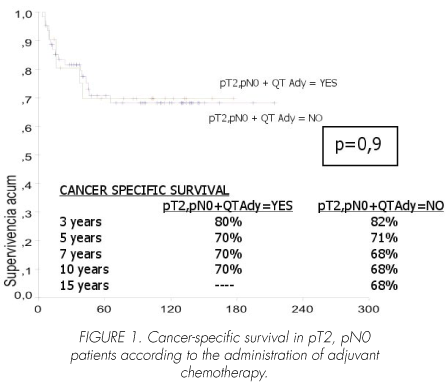
No significant differences in cancer-specific survival were seen in pT2, pN0 patients either treated or not treated with adjuvant chemotherapy (p=0.9). Mean survival in chemotherapy-treated patients was 130 (95% CI, 98-162) months, as compared with 154 (95% CI, 130-178) months in those not receiving chemotherapy (Figure 1).
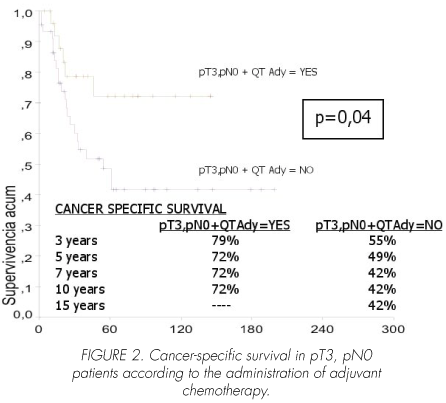
In pT3, pN0 patients, adjuvant chemotherapy significantly improved cancer-specific survival (p=0.04). Mean survival time in patients treated with chemotherapy was 111 (95% CI, 88-135) months versus 98 (95% CI, 70-126), with a median of 54 (95 CI%, 24-84) months in those receiving no chemotherapy (Figure 2).
In pT4, pN0 patients, adjuvant chemotherapy did not significantly increase cancer-related survival (p=0.6). Mean survival time in chemotherapy-treated patients was 71 (95% CI, 39-104), with a median of 20 months, versus 38 (95% CI, 17-58), and a median of 30 (95% CI, 22-38) months in those not receiving chemotherapy (Figure 3).

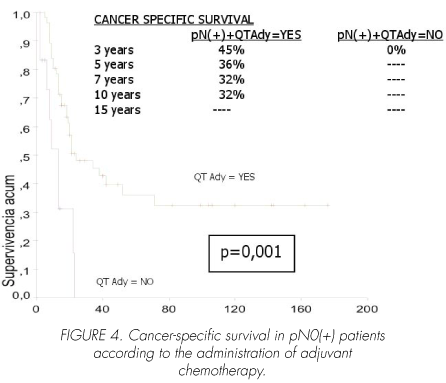
Among pN (+) patients, adjuvant chemotherapy significantly increased cancer-specific survival (p=0.001). Mean survival time in patients receiving chemotherapy was 72 (95% CI, 50-94) with a median of 24 (95% CI, 7-41) months versus 12 (95% CI, 8-17) with a median of 13 (95% CI, 8-18) months in those not treated with chemotherapy (Figure 4).
The univariate analysis (Cox regression) showed a significant association between adjuvant chemotherapy and a shorter cancer-specific survival (RR 1.452 95% CI: 1.028- 2.057 p=0.03).
In the multivariate analysis, a trend for a decrease in cancer-specific mortality was seen with chemotherapy (RR: 0.651 95% CI 0.398-1.065, p=0.08).
Discussion
Treatment with the chemotherapy schedule MVAC (methotrexate, vinblastine, adriamycin and cisplatin) was introduced in 1985, being the first to be used for metastatic bladder cancer (15). Global response rate was 72%, including a complete response rate of 36%. Since this treatment regimen was significantly active on the primary tumor, it was later added to cystectomy.
The results from our series show that chemotherapy does not influence survival in pT2, pN0 patients. Similarly, chemotherapy does not improve survival in pT4, pN0 patients, although a more extensive series may be necessary to support this statement. In contrast, we did find significant differences when chemotherapy was administered to patients with pT3, pN0 extravesical disease. Survival is significantly improved in pN+ patients treated with chemotherapy as compared with those not receiving such treatment.
The univariate analysis showed that chemotherapy-treated patients had a shorter survival (RR: 1.42), which can be easily understood since it is used in patients with a poorer prognosis. In the multivariate analysis adjuvant chemotherapy improves prognosis (RR: 0.651) with a trend for significance (p=0.08).
Despite the limitations inherent in retrospective analyses, our results have internal validity and could be extrapolated to other Spanish populations with similar socio-demographic characteristics. These results lead us to reassess our indications of adjuvant chemotherapy, and reveal a potential overtreatment in an important number of patients in our series, especially for the last two 5-year periods. Finally, our results suggest that chemotherapy should only be offered to patients pertaining to the previously described b and c risk groups.
AEU clinical practice guidelines (7) do not establish any differential features among patients with muscle-invasive disease that could help to decide on the use of chemotherapy. These guidelines also state that clear evidence of any potential benefit from adjuvant chemotherapy is still lacking, according to two reviews on this subject (16, 17). Prospective studies performed so far (18-21) suggest that chemotherapy may to be indicated in patients with a high relapse risk (pT3-pT4a and/or N+ stages). Further prospective studies are needed to specifically evaluate the indication for adjuvant chemotherapy. The benefits of adjuvant chemotherapy have only been confirmed in two of the six randomized studies that have explored this issue. The only meta-analysis evaluating adjuvant chemotherapy was performed in 491 patients from six clinical trials. Despite its power, this meta-analysis has important limitations due to the reduced patient sample, as well as to the impact of prematurely terminated studies and the number of patients not completing the assigned treatment. However, 25% of patients showed a decrease in the relative death risk favoring adjuvant chemotherapy over surgery alone (p=0.02) (22).
Conclusions
1. Adjuvant chemotherapy has not been shown to improve survival in patients with either pT0-2, pN0 (p=0.9) or pT4, pN0 stages (p=0.6). In contrast, it did increase survival in patients with pT3, pN0 extravesical disease (p=0.04), even in those with lymph node involvement (pN+) (p=0.001).
2. In the multivariate analysis, adjuvant chemotherapy only shows a trend regarding cancer-specific survival. This trend can only be defined as "protective" since it decreases cancer-specific mortality (RR=0.651, p=0.08).
 Correspondence:
Correspondence:
Juan Ignacio Monzo Gardiner
Doctor Esquerdo, 46
28007 Madrid. (Spain).
juanimonzo@hotmail.com
Accepted for publication: June 18th, 2008.
References and recomended readings (*of special interest, **of outstanding interest)
1. GLOBOCAN 2002. International Agency for research on Cancer. www.iarc.fr. Acceso Junio de 2007. [ Links ]
2. Parkin DM, Whelan SL, Ferlay J et al. Cancer incidence in five continents, Vol VIII, IARC Scientific Publications No. 155, Lyon, IARC. (eds) (2003). [ Links ]
3. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin., 2007; 57: 43-46. [ Links ]
4. Ferlay J, Bray F, Pisani P, Parkin D M, Globocan. Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0. IARC Cancer Base No 5. Lyon, IARC Press. 2000. [ Links ]
5. Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1.054 patients. J Clin Oncol, 2001; 19: 666-675. [ Links ]
*6. Sternberg CN, Donat M, Bellmut J, et al. Chemotherapy for Bladder cancer: Treatment Guidelines for Neoadjuvant Chemotherapy, bladder preservation, Adjuvant chemotherapy, and Metastatic Cancer. Urology, 2007; 69 (Supp 1A):62-79. [ Links ]
**7. Guidelines on Bladder Cancer: Muscle-invasive and metastatic. European Urology Association of Urology 2006. www.uroweb.org/fileadmin/user_upload/Guidelines. [ Links ]
8. Soloway MS, López AE, Patel J, Lu Y. Results of radical cystectomy for transitional cell carcinoma of the bladder and the effects of chemotherapy. Cancer, 1994; 76:1926-1931. [ Links ]
*9. Frazier HA, Robertson JE, Dodge RK, Paulson DF. The value of pathologic factors in predicting cancer-specific survival among patients treated with radical cystectomy for transitional cell carcinoma of the bladder and prostate. Cancer, 1993; 71:3993-4001. [ Links ]
10. Nishiyama H, Habuchi T, Watanabe J, et al. Clinical outcome of a large-scale multi-institutional retrospective study for locally advanced bladder cancer: a survey including 1131 patients treated during 1990-2000 in Japan. Eur Urol., 2003; 45:176-181. [ Links ]
11. Herr HW. Extent of surgery and pathology evaluation has an impact on bladder cancer outcome after radical cystectomy. Urology, 2003; 61:105-108. [ Links ]
12. Thieblemont C, Fendler JP, Trillet-Lenoir V, et al. Prognostic factors of survival in infiltrating urothelial bladder carcinoma. A retrospective study of 158 patients treated by radical cystectomy. Bull cancer, 1996; 83:139-146. [ Links ]
13. Takahashi A, Tsukamoto T, Tobisu K, et al. Radical cystectomy for invasive bladder cancer: results of a multi-institutional pooled analysis. Jpn J Clin Oncol, 2004; 34:14-19. [ Links ]
14. Cheng L, Weaver AL, Leibivich BC, et al. Predicting the survival of bladder carcinoma in patients treated with radical cystectomy. Cancer, 2000; 88:2326-2332. [ Links ]
15. Sternberg DL, Yagoda A, Scher HI, et al. Preliminary results of M-VAC for transitional cell carcinoma of urothelium. J Urol, 1985; 133:403-407. [ Links ]
*16. Herr HW, Dotan Z, Donat MD, et al. Defining optimal therapy for muscle invasive bladder cancer. J Urol., 2007; 177:437-442. [ Links ]
*17. Rosenberg JE, Carroll PR, Small EJ. Update on chemotherapy for advanced bladder cancer. J Urol., 2005; 174:14-20. [ Links ]
18. Studer UE, Bacchi M, Biedermann C, et al. Adjuvant cisplatin chemotherapy following cystectomy for bladder cancer: results of prospective randomized trial. J Urol., 1994; 152:81-84. [ Links ]
19. Skinner DG, Daniels JR, Rusell CA, et al. The role of adjuvant chemotherapy following cystectomy for invasive bladder cancer: a prospective comparative trial. J Urol., 1991; 145:459-464. [ Links ]
20. Stockle M, Meyenburg W, Wellek S, et al. Advanced bladder cancer (stages pT3b, pT4a, pN1 and pN2): improved survival after radical cystectomy and 3 adjuvant cycles of chemotherapy. Results of a controlled prospective study. J Urol., 1992; 148:302-306. [ Links ]
*21. Freiha F, Reese J, Torti FM. A randomized trial of radical cystectomy versus radical cystectomy plus cisplatin, vinblastine and methrotrexate for muscle invasive bladder cancer. J Urol., 1996; 155:495-499. [ Links ]
*22. Advanced Bladder Cancer (ABC) Meta -Analysis Collaboration. Adjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data. Eur Urol., 2005; 48:199-201. [ Links ]











 texto en
texto en 


