Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nefrología (Madrid)
versión On-line ISSN 1989-2284versión impresa ISSN 0211-6995
Nefrología (Madr.) vol.29 no.5 Cantabria 2009
Modification of Diet in Renal Disease equation in the risk stratification of contrast induced acute kidney injury in hospital inpatients
T. Erselcan1, Z. Hasbek1, I. Tandogan2, C. Gumus3, I. Akkurt4
1Department of Nuclear Medicine. Cumhuriyet University School of Medicine. Sivas (Turkey)
2Department of Cardiology. Cumhuriyet University School of Medicine. Sivas (Turkey)
3Department of Radiology. Cumhuriyet University School of Medicine. Sivas (Turkey)
4Department of Pulmonary Medicine. Cumhuriyet University School of Medicine. Sivas (Turkey)
Dirección para correspondencia
ABSTRACT
Background: Several organizations recommend using estimated glomerular filtration rate (eGFR) in kidney function monitoring, preferably calculated with Modification of Diet in Renal Disease (MDRD) formula. The role of this formula is not clear in the risk stratification of contrast induced acute kidney injury (CIAKI) in nonsteady state patients.
Aim: Comparative evaluation of the MDRD eGFR in risk stratification of CIAKI.
Method: GFR was measured twice (preand post-examination) by Tc-99m-DTPA, along with serum levels of urea nitrogen and creatinine in 32 patients (mean age ± SD; 60.1 ± 13.2 years) needing hospital care for various reasons and underwent to x-ray examination with contrast media (mean; 90.2 ± 16.8 ml). eGFR was calculated by the dedicated formula. Agreement between measured GFR (mGFR) and MDRD eGFR was assessed and patients were scored and stratified for CIAKI by using first mGFR, then eGFR and results were compared.
Results: A moderate correlation was obtained between mGFR and eGFR (r = 0.47, p < 0.001) and the difference was not significant. However, Bland&Altman analysis revealed large limits of agreement between mGFR and eGFR (-80.3 to 55.2) with a mean difference of -12.5 ml/min/1.73m2. In ROC analysis, when mGFR values were classified as normal (> 60 ml/min/1.73m2) and decreased (<60ml/min/1.73m2), AUC was 0.80 (95%CI; 0.62-0.92) for eGFR, with a sensitivity of 29% and specificity of 100%. Furthermore, the risk group categorization, using eGFR instead of mGFR was resulted in a group change for four patients (13%); from moderate to low risk group.
Conclusion: It seems that MDRD eGFR differs from mGFR. In nonsteady state patients CIAKI classification using eGFR should be considered with caution.
Key Words: Contrast nephropathy, glomerular filtration rate-risk stratification, Tc-99m DTPA, acute kidney injury.
RESUMEN
Antecedentes: Varios organismos recomiendan el uso de la tasa de filtrado glomerular estimada (TFGe) en la monitorización de la función renal, calculada preferentemente con la fórmula de Modificación de la Dieta en la Enfermedad Renal (MDRD). El papel de esta fórmula no está claro en la estratificación del riesgo de la lesión renal aguda inducida por contraste en pacientes no estables.
Objetivo: Evaluación comparativa de la TFGe de la MDRD en la estratificación del riesgo de lesión renal aguda inducida por contraste. Método: La tasa de filtrado glomerular (TFG) se midió dos veces (pre- y posexamen) mediante Tc-99m-DTPA, junto con los niveles de nitrógeno ureico en suero y creatinina en 32 pacientes (edad media ± DE; 60,1 ± 13,2 años) que precisaban de cuidados hospitalarios por diversas razones y que se sometieron a rayos- x mediante contraste (mediana; 90,2 ± 16,8 ml). La TFGe se calculó mediante la fórmula correspondiente. Se evaluó la concordancia entre la TFG medida (TFGm) y la TFGe de la MDRD, asignando a los pacientes un baremo de estratificación para la lesión renal aguda inducida por contraste, usando primero la TFGm y posteriormente TFGe, comparando los resultados.
Resultados: Se obtuvo una correlación moderada entre la TFGm y la TFGe (r = 0,47, p < 0,001), con una diferencia no significativa. Sin embargo, el análisis de Bland&Altman reveló grandes límites de concordancia entre la TFGm y la TFGe (-80,3 a 55,2), con una diferencia media de -12,5 ml/min/1,73 m2. En el análisis por método ROC, cuando los valores de la TFGm se catalogaron como normales (>60 ml/min/1,73 m2) y disminuidos (< 60 ml/min/1,73 m2), el área bajo la curva fue 0.80 (CI 95%; 0,62-0,92) para TFGe, con una sensibilidad del 29% y una especificidad del 100%. Es más, la categorización del grupo de riesgo, utilizando la TFGe en lugar de la TFGm, derivó en un cambio de grupo para cuatro pacientes (13%), del grupo de riesgo moderado al de riesgo bajo.
Conclusión: Parece que la TFGe medida mediante MDRD difiere de la TFGm, por lo que en pacientes no estables la clasificación de la lesión renal aguda inducida por contraste mediante TFGe se debe analizar con precaución.
Palabras clave: Nefropatía de contraste, Índice de estratificación del riesgo de filtrado glomerular, Tc-99m-DTPA, Lesión renal aguda.
Introduction
In the last 30 years, there has been a marked increase in diagnostic and interventional procedures in which iodinated contrast media (CM) is in use1. Iodinated CMs are excreted mainly by glomerular filtration2. There is thus, a significant correlation between both body and renal clearances of contrast media and glomerular filtration rate3.
CM induced nephropathy or with a contemporary term "contrast induced acute kidney injury" (CIAKI) is a wellknown and common cause of hospital-acquired renal failure. Although the clinical significance is variable, several studies have demonstrated markedly increased mortality, even up to 27%4. Therefore, it is imperative to identify patients at risk for this disease. The definition of CIAKI includes absolute (>0.5mg/dl) or relative increase (> 25%) in serum creatinine (sCr) at 48-72 h after exposure to a contrast agent compared to baseline serum creatinine values, when alternative explanations for renal impairment have been excluded5.
Recently, practical risk scoring systems have been proposed for the prevention of CIAKI instead of only screening patients with sCr. One of the major contributors in these scoring systems is naturally the level of renal function. Measurement of the glomerular filtration rate (GFR) is the best sensitive test to assess the renal function, but is not easy to obtain6. Instead, several authors and organizations support using estimated glomerular filtration rate (eGFR; estimated GFR from sCr by a regression formula), preferably calculated with Modification of Diet in Renal Disease (MDRD) formula7,8. Although, MDRD eGFR has been developed in a population consisting predominantly of patients with chronic kidney disease (CKD) and reduced GFR, it has also been applied in patient populations with mostly normal kidney function, including CIAKI followups9. Moreover, some authors have found eGFR to be one of the significant risk markers in the development of CIAKI9,10.
We hypothesized that MDRD eGFR might be limited in patients with rapidly changing kidney function, such as with acute coronary artery disease, unregulated DM or unregulated hypertension, nevertheless candidate to CM exposure and carriying the risk of CIAKI in various levels. Hence, the primary objective of the study was to evaluate individual role of the MDRD eGFR in the risk stratification of CIAKI in such patients. First, we sought agreement between measured GFR (mGFR) and the MDRD eGFR. Next, patients were classified for CIAKI using first eGFR and then mGFR values and results were compared.
Materials and methods
Patients
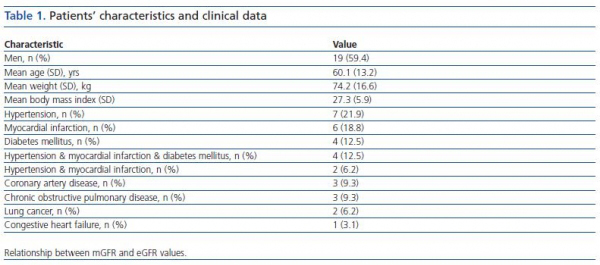
In this prospective study, 32 consecutive Caucasians patients (19 men, 13 women), who had been hospitalized within 3 recent days and candidate to have an X-ray examination with iodinated CM were included into the study. Their common characteristic was being in nonsteady state condition and necessitating hospital care due to various clinical problems (table 1). Criteria of nonsteady state condition, as were accepted in the present work were having unregulated hypertension or unregulated glycemia or myocardial infarction or arrithmia at hospital admission. Mean hospitalization time was 2.2 ± 0.4 days prior to pre-CM application. Patients recently (within two months) received CM or known to have chronic renal diseases or age < 18 years were omitted. No modification has been made in individual treatment protocol of any patient for this study. No particular prophylactic medication was used prior to CM application, other than routine hydration with intravenous administration of 500 ml NaCl 0.9% before and after injection of the contrast agent.
The Ethics Committee of Faculty approved the study and written informed consent was obtained from all patients.
X-ray examinations were cardiac catheterization in 18 patients and tomography in 14 patients, realized by using one of non-ionic monomeric type CM (iopromide, iopamidol or iomeprol). Mean CM volume was; 90.2 ± 16.8 ml (range: 60-145 ml).
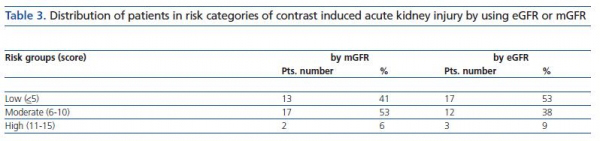
Pre-risk scoring for CIAKI was realized by using a method published by Mehran et al.9. In short, patients were scored according to the pre-defined variables, such as age, diabetes mellitus, anemia, hypotension, CM volume, mGFR, as described in the method. Next, patients were categorized as low risk (score < 5), moderate risk (6-10) and high risk (11-15) by using first mGFR, then eGFR for the score calculation.
GFR measurement
Plasma clearance of diethylene triamine pentaacetic acid (DTPA), labeled with Tc-99m was realized in GFR measurements by using two blood samples and slopeintercept method, before and after CM applications (at 48 h). Tc-99m DTPA was prepared with fresh pertechnetate and a current DTPA kit (Pentacis®, CIS bio international, France). The Tc-99m DTPA labeling efficiency was greater than 98%. Following its I.V. administration (37 MBq) blood samples (5 ml) were taken at 2 and 4 hours to heparinized syringe, plasma was separated and radioactivity was assayed in a well type gamma counter (Berthold, Germany). Then, the absolute measured GFR values were obtained using the following equation.
Clearance(Tc-99m DTPA) = D x ln(P1/P2) / T2-T1 x exp (T1 ln P2)-(T2 ln P1) / T2-T1
Where, D = injected dose (counts/min); P1 = plasma activity at T1 (counts/min/ml); P2 = plasma activity at T2 (counts/min/ml).
Those values were normalized to body surface area and corrected according to Brochner-Mortensen method11-13.
Precision of the method, by means of coefficient of variation (CV) was 3.4%. It was calculated by measurement twice GFR in three healthy volunteers within 48 hours.
SCr concentrations were measured in each patient before and after X-ray examination by colorimetric analysis, based on the Jaffe rate (alkaline picrate) method using autoanalyzer (Beckman Coulter, Synchron LX20, USA). Analytical range was 0.1-25 mg/dl (8.84-2210 µmol/L) and the mid-term (6 months) precision (CV) of low and high control values of the laboratory were 6.2 % and 4.3%, respectively.
eGFR calculation; eGFR was calculated using the modified MDRD formula as; eGFR (mL/min/1.73 m2) = 175 x (creatinine, mg/dl)-1.154 x (Age, years.)-0.203 (x 0.742 if female)14,15.
Statistical analysis
Non-parametric two related samples test (Wilcoxon) was used in comparison of pre- and post-CM serum parameters, mGFR and eGFR values. Relationship and method agreement between mGFR and eGFR was sought by linear correlation, regression and Bland & Altman analysis, respectively. The receiver operating characteristic (ROC) analysis was used to test sensitivity and specificity of eGFR to discriminate patients with decreased renal function (mGFR < 60 ml/min/1.73 m2). Chi-square test with Yates' correction for continuity was used for comparison of risk categories of CIAKI by using eGFR or mGFR. Significance level was set to p <0.05 for all tests.
Results
Being mostly at the beginning of the hospitalization period, pre-CM as well as post-CM mGFR values were dispersed in the study group with a wide range of renal function, from 22.7 to 179.8 ml/min/1.73m2 and from 27.9 to 175.9 ml/min/1.73m2, respectively. Three patients had pre-CM sCr >1.4 mg/dl, where as 14 patients had mGFR < 60 ml/min/1.73 m2. However, pre-CM sCr was between 0.8 and 1.2 mg/dl in 60% of patients.
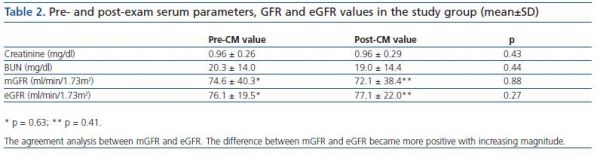
Mean values of sCr, BUN, mGFR and eGFR were shown in the table 2. At first look, comparison of pre- and post-CM values of sCr, BUN, mGFR and eGFR did not yield statistically significant differences, most probably due to high SD values (Wilcoxon paired rank test). Comparison of mean mGFR with eGFR values did not show differences as well (table 2).
Statistically significant, but a moderate linear relationship was observed between pre-CM mGFR and eGFR (r = 0.47, p < 0.001). When mGFR defined as dependent variable, the regression equation was y = 0.72 x + 12.8, with R2 = 0.23 (p < 0.001) (figure 1). Post-CM relationship was very similar with y = 0.62 x + 17.5 and R2 = 0.25 (p <0.001).
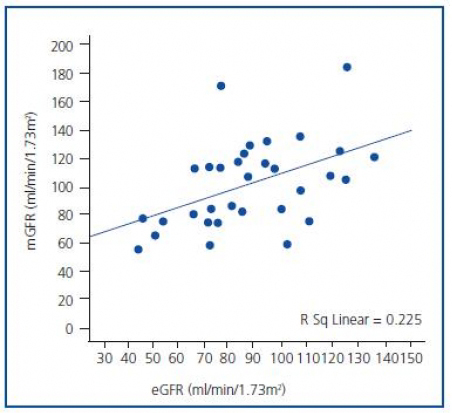
Figure 1. Relationship between mGFR and eGFR values.
The agreement between two methods was evaluated by Bland and Altman analysis, which showed large limits of agreement between mGFR and eGFR (-80.3 to 55.2) with a mean difference of -12.5 ml/min/1.73 m2 (figure 2). This means that any value of mGFR would be estimated as 80.3 ml less or 55.2 ml more by eGFR. On the other hand, disagreement was a type of proportional, as the difference was significantly related to the magnitude of estimation; eGFR overestimated mGFR at lower degree of renal function, while underestimated higher values.
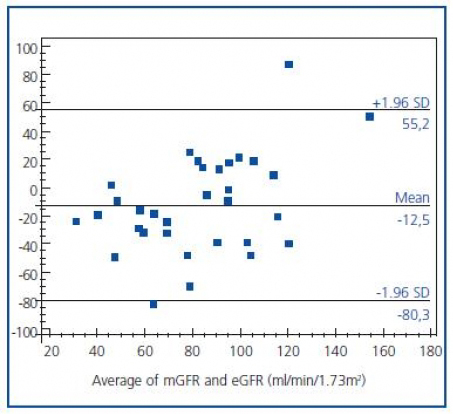
Figure 2.The agreement analysis between mGFR and eGFR. The difference
between mGFR and eGFR became more positive with increasing magnitude.
The ability of MDRD eGFR to discriminate a decrease in renal function was assessed by ROC analysis. When mGFR = 60 ml/min/1.73 m2 was selected as a cutoff value, AUC for eGFR was 0.80 (95% CI, 0.62-0.92). The eGFR has a sensitivity of 29% and a specificity of 100% in detecting patients with a decreased renal function, as demonstrated in figure 3. The pre-exam mGFR <60 ml was calculated as if >60 ml in ten cases (10/14, 71%) with the eGFR formula.
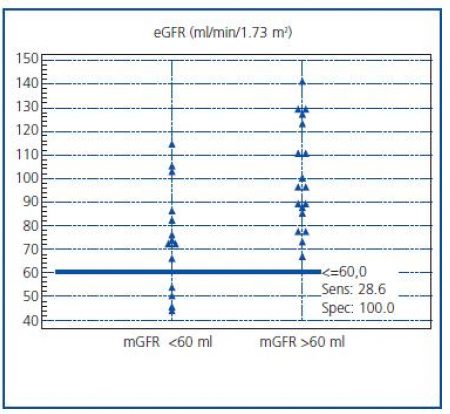
Figure 3. Distribution of eGFR values according to the cut-off value
of 60 ml/min/1.73 m2 of mGFR.
Range of the CIAKI risk score in the study group was 1 to 13. The distribution of patients according to CIAKI risk categories is shown in table 3. There were 13 patients in low, 17 in moderate and 2 in high risk group when taking in consideration mGFR values. Re-stratification using eGFR was resulted in a group change for four patients; from moderate risk to low risk group. One patient switched also from moderate to high risk group (table 3). Yet, the difference between mGFR and eGFR risk groups did not attain to a significant level by Chi-square test (p = 0.45).
On the other hand, more than 0.5 mg/dl increase in sCr was recorded in two patients after CM application. According to common definition, these patients had CIAKI. However, mGFR values were stable. Common features of them were both were using diuretics and anti-aggregant (salicylic acid) and had significantly decreased pre-CM mGFR values. First patient was hospitalized for unregulated hypertension; her pre- and post-CM sCr values were 0.70 and 1.60 mg/dl (BUN; 10 and 12 mg/dl), but mGFR values were comparable as 43.4 and 49.9 ml/min/1.73 m2, respectively. eGFR values were 114.3 and 44.0 ml/min/1.73 m2. Second patient was azotemic (BUN; 45 and 77 mg/dl) with unregulated diabetes mellitus and hypertension. While pre-exam vs. post-exam sCr and eGFR values were 1.00 vs. 1.60 mg/dl and 72.9 vs. 42.4 ml/min/1.73 m2, respectively, mGFR remained again relatively constant as 22.7 vs. 31.5 ml/min/1.73 m2. Followup of sCr and BUN revealed fluctuations and normalized by the end of their hospital stay and they did not need hemodialysis. Both patients left the hospital within a week and did not have abnormal sCr values in 6 months in their routine examinations. Risk score of first patient was 3 (belonging to low risk group) and second patient's was 8 (moderate risk group).
Discussion
The present study suggests that eGFR MDRD may cause erroneous CIAKI classification in nonsteady state patients. CIAKI is an important adverse event of diagnostic and therapeutic intravascular application of iodinated contrast agents. In the general population, the incidence of CIAKI is estimated to be 1% to 6%16. However, the risk may be as high as 50% in some patient subgroups. Such as, patients with diabetes and pre-existing renal impairment are at high risk, and CIAKI incidence increases in patients with multiple comorbidities1,16,17. On the other hand, recent improvements in contrast agents and radiological imaging tools have resulted in an increasing number of patients undergoing contrast media-enhanced examinations, either they are at steady state or not. Approximately 80 million doses of iodinated CM were prescribed worldwide in 2003, probably making CM one of the most commonly used medications18. Thus, in recent years predictor models have been proposed to determine risks of CIAKI. We have used one of them, a pre-risk scoring system, published by Mehran et al.9. They found that contrast-medium nephropathy was strongly associated with an increased risk score: the incidence was 7.5% among patients with a low score and 57.3% among those with a high risk score. Although this method was based on a patient population who had undergone to percutaneous coronary intervention (like some of our patients in coronary angiography), 90% of the patients in that study had sCr < 1.5 mg/dl, so did our patients. This risk scoring system was chosen especially because of the studied patient population in the model, with almost free of chronic kidney disease.
Among the stated individual risk factors (patient age, diabetes, hypotension, anemia, cardiac heart failure, CM volume, level of sCR) decreased renal function was remarked to be the most significant one8. Common approach to assess renal function before administration of CM is to obtain sCr. This is probably because of the positive correlation between the dose of CM and the rise in sCr19.
On the other hand, eGFR has been recommended instead of sCr in the National Kidney Foundation guidelines, indicating that it normalizes at the same time renal function for total muscle mass. It was also stated that measurement of creatinine clearance using a timed (e.g., 24-hour) urine collection for assessment of the GFR is not more reliable than estimation using a prediction equation.7 Hence, there has been extensive work on a noninvasive and accurate estimation method of GFR in recent years. A number of easy-to-use mathematical equations, incorporating different anthropometrical variables in addition to biological parameters, have been developed to predict GFR. The MDRD study equation was developed in 199920. The original equation was; eGFR = 186 x (sCr)-1.154 x (age)-0.203 x 0.742 (if the subject is female) or x1.212 (if the subject is black). This equation was reexpressed in 2005 for use with a standardized serum creatinine assay, as eGFR = 175 x (standardized sCr) -1.154x(age)-0.203 x 0.742 (if the subject is female) or x 1.212 (if the subject is black)15. MDRD eGFR formula has been shown to work well in patients at steady state. Indeed, it was derived from patients with chronic kidney disease who were at steady state (mean GFR, 40 ml/min/1.73 m2) and had renal functional changes over several months and years. However, renal function may show fluctuation in recovery, nonsteady state patients along with the cardiovascular dynamics.
Our aim was not to assess accuracy of the MDRD eGFR, but to observe, although in a small scale, its impact on the risk stratification of CIAKI in nonsteady state patients. We have not met a similar published data in the literature. In this respect, we first evaluated the method agreement between mGFR and eGFR in patients needing hospital care, since CM receiving patients are not only out patients or free of any renal or cardiac dysfunctions. Secondly, we compared performance of MDRD eGFR in identification of patients with decreased renal function. A moderate relationship was observed between mGFR and eGFR (r = 0.47) and it seemed that there was a disagreement between two methods in this patient population. Poggio et al. also reported that MDRD eGFR would have cause erroneous results in nonsteady state conditions inherent in acute kidney insufficiency21. However, our patient population differered in the sense that none of them had acute kidney insufficiency before CM receiving.
In general, prediction formulas (or regression equations) always carry more or less bias depending on the study population. Hence, it is not surprising to see contradictory reports in the literature. In a recent meta-analysis, it was concluded that MDRD eGFR equation showed little bias for GFR estimates < 60 ml/min/1.73 m2 in the pooled data base, underestimated mGFR for levels of eGFR between 60 and 119 ml/min/1.73 m2, and overestimated mGFR for levels of eGFR >120 ml/min/1.73 m2 (as compared to I-125 iothalamate clearance)22. Another report concluded that MDRD eGFR underestimated mGFR (by Tc-99m DTPA clearance) in Chinese patients with near-normal renal function and overestimated in patients with chronic kidney disease stages 4-523. In the present study, it can be seen from Bland&Altman analysis (figure 2) that eGFR underestimated mGFR values in normal ranges and overestimated in lower rates. Overestimation of GFR in lower rates led to insufficient discrimination of patients with decreased renal function, as it can be seen in the ROC analysis (figure 3). This also led to misclassification of some patients for CIAKI (Table 3). One of the working group reported that the risk of CIAKI is elevated and becomes clinically important when the baseline serum creatinine level is >1.3 mg/dl (>114.9 µmol/L) in men and >1.0 mg/dl (>88.4 µmol/L) in women, equivalent to eGFR >60 ml/min per 1.73m2. 8 When we selected eGFR < 60 ml/min as a cutoff value in the present study, four patients have been classified as having low risk instead of moderate risk.
Clinical presentation in CIAKI is mostly asymptomatic, but in some patients acute renal failure with necessity of hemodialysis can occur. In the present study two patients had CIAKI by definition. However, mGFR was relatively stable, but decreased, although both had normal sCr values before CM administration. It is known that in case of significantly decreased GFR, sCr secretion increases, causing apparently a normal sCr level. Another explanation for this discrepancy would be that both patients were using salicylic acid, which can inhibit secretion of creatinine by the proximal tubule24. Other than salicylates, several drugs, such as cimetidine, trimethoprim and pyrimethamine have been reported to increase plasma creatinine by the same mechanism without influencing its glomerular filtration24. Finally, MDRD eGFR has been developed chiefly in a populations consisting predominantly of patients with chronic kidney disease (CKD) and reduced GFR. However, none of our patients had the diagnosis of CKD prior to CM application.
Study limitations; The present study was realized with a limited number of patients due to ethical reasons as GFR measurement by Tc-99m-DTPA which is not a commonly accepted indication for the CIAKI follow ups. This has led to even small numbers of patients in the CIAKI risk groups and hampered the statistical significance. On the other hand, the renal function could have been impacted in different degrees by drugs or the concurrent illness including acute myocardial infarction, anemia, etc. in the study population. However, this type of patients will always have X-ray examinations with contrast exposure. This fact reminds a need for better real time biomarkers of renal function in such patients and one may have at least an idea about the place of MDRD eGFR in the follow up of non steady state patients owing to the present study results.
Conclusion
It seems that MDRD eGFR differs from mGFR. In nonsteady state patients CIAKI classification using eGFR should be considered with caution.
References
1. Waybill MM, Waybill PN. Contrast media-induced nephrotoxicity: identification of patients at risk and algorithms for prevention. J Vasc Interv Radiol 2001;12:3-9. [ Links ]
2. Persson PB, Hansell P, Liss P. Pathophysiology of contrast mediuminduced nephropathy. Kidney Int 2005;68:14-22. [ Links ]
3. Morcos SK, Brown PW, Oldroyd S, el Nahas AM, Haylor J. Relationship between the diuretic effect of radiocontrast media and their ability to increase renal vascular resistance. Br J Radiol 1995;68:850-53. [ Links ]
4. Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA 1996;275:1489-94. [ Links ]
5. Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology and patients at risk. Kidney Int Suppl. 2006;100:S11-5. [ Links ]
6. Thomsen HS, Morcos SK. Contrast media and the kidney: European Society of Urogenital Radiology (ESUR) Guidelines. Br J Radiol 2003;76:513-18. [ Links ]
7. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(Suppl 1):S1-266. [ Links ]
8. Lameire N, Adam A, Becker CR, et al. CIN Consensus Working Panel. Baseline renal function screening. Am J Cardiol 2006;98(6A):21K-6K. [ Links ]
9. Mehran R, Aymong ED, Nikolsky E et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004;44:1393-9. [ Links ]
10. Brown JR, DeVries JT, Piper WD et al. Northern New England Cardiovascular Disease Study Group. Serious renal dysfunction after percutaneous coronary interventions can be predicted. Am Heart J 2008;155:260-6. [ Links ]
11. Blaufox MD, Aurell M, Bubeck B, et al. Report of the Radionuclides in Nephrourology Committee on renal clearance. J Nucl Med 1996;37:1883-90. [ Links ]
12. Piepsz A, Colarinha P, Gordon I, et al. Paediatric Committee of the European Association of Nuclear Medicine. Guidelines for glomerular filtration rate determination in children. Eur J Nucl Med 2001;28: BP31-6. [ Links ]
13. Fleming JS, Zivanovic MA, Blake GM et al. British Nuclear Medicine Society. Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun 2004;25:759-69. [ Links ]
14. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247-54. [ Links ]
15. Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53:766-72. [ Links ]
16. Parfrey P. The clinical epidemiology of contrast-induced nephropathy. Cardiovasc Interv Radiol 2005;28(Suppl 2):S3-11. [ Links ]
17. Goldenberg I, Matetzky S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. CMAJ 2005;172:1461-71. [ Links ]
18. Katzberg RW, Haller C. Contrast-induced nephrotoxicity: clinical landscape. Kidney Int Supp. 2006;100:S3-7. [ Links ]
19. Rosovsky MA, Rusinek H, Berenstein A, Basak S, Setton A, Nelson PK. High-dose administration of nonionic contrast media: a retrospective review. Radiology 1996;200:119-22. [ Links ]
20. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. modification of diet in renal disease study group. Ann Intern Med 1999;130:461-70. [ Links ]
21. Poggio ED, Nef PC, Wang X, et al. Performance of the Cockcroft-Gault and modification of diet in renal disease equations in estimating GFR in ill hospitalized patients. Am J Kidney Dis 2005;46:242-52. [ Links ]
22. Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol 2007;18:2749-57. [ Links ]
23. Zuo L, Ma YC, Zhou YH, Wang M, Xu GB, Wang HY. Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis 2005;45:463-72. [ Links ]
24. Andreev E, Koopman M, Arisz L. A rise in plasma creatinine that is not a sign of renal failure: which drugs can be responsible? J Intern Med 1999;246:247-52. [ Links ]
![]() Correspondence:
Correspondence:
Taner Erselcan,
Cumhuriyet University School of Medicine
Department of Nuclear Medicine
Sivas (Turkey)
terselcan@yahoo.es