My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nefrología (Madrid)
On-line version ISSN 1989-2284Print version ISSN 0211-6995
Nefrología (Madr.) vol.32 n.2 Cantabria 2012
Lupus-related podocytopathy. Could it be a new entity within the spectrum of lupus nephritis?
Silvina Gutiérrez, Juan P. Petiti, Ana I. De-Paul, Alicia I. Torres, Jorge H. Mukdsi
Centro de Microscopía Electrónica. Facultad de Ciencias Médicas. Universidad Nacional de Córdoba. Córdoba (Argentina)
The classification of lupus nephritis was revised by the ISN/RPS in 2003. The increasingly recognized phenomenon of apparent minimal change disease (MCD) in the context of systemic lupus erythematosus (SLE), is not accepted in the above classification and is associated to a recent new pathological entity called lupus podocitopathy.1 A 32-year-old caucasian woman presented with arthralgia and swelling of the face, hands, and legs. Physical exam revealed pretibial edema and a patch of skin thickening on the left flank, consistent with morphea. Blood presure was 130/70mmHg; proteinuria 4.5g/dl; serum creatinine 0.9mg/dl; and albumin 2 g/dl. Urinalysis revealed fat casts. Serology was negative for hepatitis B, C, HIV-1 and HIV-2. ANA titer was 1/1300, C3 70mg/dl and anti ds-DNA was elevated. There was no history of nonsteroidal anti-inflammatory drug use in the patient. A diagnosis of SLE was made. Sections from the needle renal biopsy showed cortex with 10 normocellular glomeruli with mild mesangial hypercellularity and mesangial matrix increased. There were no evident tubular, interstitial, and vascular lesions (Figure 1 A). Immunofluorescence microscopy revealed mesangial granular deposition of IgG (2+) (Figure 1 B), IgA (1+), IgM (1+), C3 (2+) (Figure 1 C) and C1q (3+) (Figure 1 D). Ultrastructural analysis showed diffuse effacement (~80%) of the epithelial cell food processes and vacuoles (Figure 2 A). Moreover few electron-dense deposits were noted in mildly expanded mesangium (Figure 2 B). Subepithelial or subendothelial deposits were not observed in the biopsy. Numerous tubulorreticular inclusions within endothelial cells of glomerular capillary were also seen (Figure 2 C). A diagnosis of lupus podocytopathy and lupus nephritis Class I (ISN/RPS) was made. Of particular interest is the podocyte involvement in different types of lupus glomerulonephritis. For example, patients with non-nephrotic proteinuria and lupus nephritis Class I and II (ISN-RPS) have not revealed significant evidence of effacement of the foot processes. Nevertheless, some adult and children show minimal or proliferative mesangial lupus nephritis and nephrotic proteinuria without peripheral immune complex, exhibiting extensive podocyte effacement, consistent with lupus podocytopathy1. It is difficult to propose an exact pathogenic mechanism for this lesion given that immune deposits are no detected in glomerular basement membrane, even though it has been hypothesized different mechanisms. Abnormal release of IL-13 from aberrant T cell2, crosstalk between renal dendritic cells and Th cells3 may directly damage to podocytes. Our patient was treated with high-dose prednisone. Six month later she remained normotensive, had no edema, with normal serum creatinine and decreased urinary protein excretion (0,5g/d). In agreement with this result Kraft et al1 have shown a significant reduction in proteinuria at last follow-up. Therefore, the podocytopathy in the SLE context responded to oral corticosteroids, remarking the important therapeutic implications of the diagnosis of this particular entity. In summary, lupus podocytopathy has become an intersting point both clinical discussion and futures investigations about the role of podocyte and it should be added to the classification of lupus nephritis.
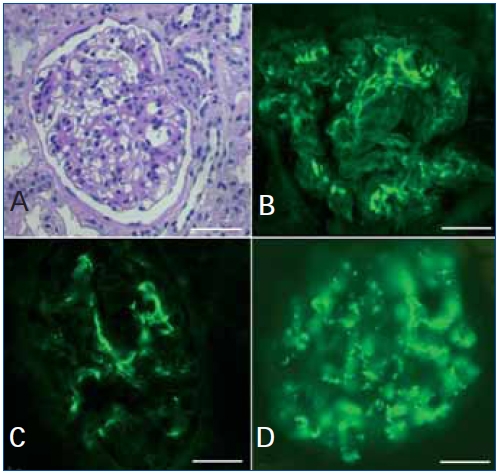
Figure 1. Light microscopy and immunofluorescence findings
(A) There are mild mesangial hypercellularity and variable increase in mesangial matrix (PAS).
Immunofluorescence microscopy shows deposits of IgG (B); C3 (C) and C1q (D) confined to the mesangium. Bars=25μm.
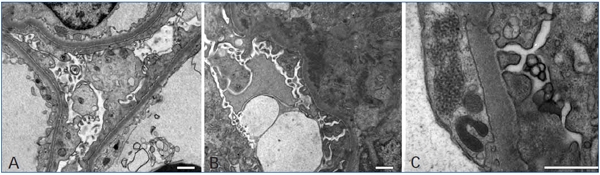
Figure 2. Ultrastructural findings
(A) Electron micrograph of a portion of a glomerulus showing diffuse foot process effacement
and microvillous transformation. (B) The electron micrograph shows paramesangial electron-dense
deposits within a mildly expanded mesangium. (C) Tubuloreticular inclusions within the endothelial cell. Bars=1μm.
Conflict of interest
The authors declare they have no potential conflicts of interest related to the contents of this article.
References
1. Kraft SW, Schwartz MM, Korbet SM, Lewis EJ. Glomerular podocytopathy in patients with systemic lupus erythematosus. J Am Soc Nephrol 2005;16:175-9. [ Links ]
2. Cunard R, Kelly CJ. T cells and minimal change disease. J Am Soc Nephrol 2002;13:1409-11. [ Links ]
3. Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, Hammerich L, Panzer U, Kaden S, et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest 2009;119:1286-97. [ Links ]
![]() Correspondence:
Correspondence:
Silvina Gutiérrez,
Centro de Microscopía Electrónica.
Facultad de Ciencias Médicas.
Universidad Nacional de Córdoba, Córdoba, Argentina
E-mail: jmukdsi@cmefcm.uncor.edu
E-mail: mukdsijorge@hotmail.com
Send to review: 25 Set. 2011
Accepted: 9 Nov. 2011














