My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nefrología (Madrid)
On-line version ISSN 1989-2284Print version ISSN 0211-6995
Nefrología (Madr.) vol.33 n.5 Cantabria 2013
https://dx.doi.org/10.3265/Nefrologia.pre2013.May.12036
Carotid Artery Function in Children with Idiopathic Nephrotic Syndrome
Función de la arteria carótida en niños con síndrome nefrótico idiopático
Nakysa Hooman1, Roya Isa-Tafreshi2, Hasan Otukesh1, Seyed-Hassan Mostafavi3, Farideh Hallaji3
1Pediatric Nephrology. Pediatric Transplantation and Dialysis Research Center (PTDRC), Ali-Asghar children hospital, Iran University of Medical Sciences. Tehran (Iran)
2Pediatric Cardiology. Ali Asghar children hospital, Iran University of Medical Sciences. Tehran (Iran)
3Pediatric Radiology. Department of Radiology, Ali-Asghar children hospital, Iran University of Medical Sciences. Tehran (Iran)
The study was supported by Iran University of medical Sciences grant number of 1039 dated 2009
Some part of this study has been presented as poster in 15th IPNA congress 29 April-2 September 2010 held in New York.21
ABSTRACT
Background: Nephrotic patients are prone to atherosclerosis in consequence of frequent exposures to hyperlipidemia, hypertension, and immunosuppressive drugs.
Objectives: We studied the carotid parameters as early indicators of atherosclerosis in children with nephrotic syndrome.
Methods: Between 2008 and 2011, 51 children with history of nephrotic syndrome enrolled in the study. The inclusion criteria were: idiopathic nephrotic syndrome with normal serum complement, at least one year after initiation of disease, glomerular filtration rate more than 20mL/min/1.73m2, age over two years old at the time of study. Seventy-five healthy sex-age-matched children considered as a control group. Carotid function parameters and left ventricular mass index were studied in nephrotic children.
Results: Steroid sensitive, resistant, and dependent nephrotic syndrome included one-third each. The mean carotid intima-media thickness (mm) in nephrotic children was 0.42 (±.14) while the mean cIMT in controls was 0.37 (±.08) (p-value <.05). After log transformation, General Linear Multivariate analysis revealed significant difference of carotid intima-media thickness in nephrotic patients (p-value <.001). Subsequently, the factor that influenced on cIMT was duration of disease (P<.05). One-half of nephrotic children who had echocardiography, showed left ventricular hypertrophy. It was correlated with carotid stiffness and systolic hypertension (P<.05).
Conclusions: Carotid intima-media thickness was thicker in nephrotic children. Carotid parameters were influenced by duration of disease and hypertension.
Key words: Nephrotic Syndrome, Idiopathic, Child, Hypertrophy, Left Ventricular, Carotid Intima- Media Thickness.
RESUMEN
Antecedentes: Los pacientes con síndrome nefrótico son propensos a sufrir aterosclerosis como consecuencia de las frecuentes exposiciones a medicamentos para la hiperlipidemia, la hipertensión e inmunodepresores.
Objetivos: Hemos estudiado los parámetros de la carótida como indicadores tempranos de aterosclerosis en niños con síndrome nefrótico.
Métodos: 51 niños con antecedentes de síndrome nefrótico participaron en el estudio entre 2008 y 2011. Los criterios de inclusión fueron: síndrome nefrótico idiopático con complemento sérico normal, al menos un año después del comienzo de la enfermedad, índice de filtración glomerular superior a 20 ml/min/1,73 m2, mayor de dos años de edad en el momento del estudio. Se tuvo en consideración a setenta y cinco niños del mismo sexo y edad como grupo de control. Se estudiaron los parámetros de la función carótida y el índice de masa ventricular izquierda en niños con síndrome nefrótico.
Resultados: Síndrome nefrótico sensible a esteroides, resistente a esteroides y dependiente de esteroides a partes iguales. El grosor íntima-media carotídeo medio (mm) en niños con síndrome nefrótico fue de 0,42 (±0,14), mientras que la TMIR media en controles fue de 0,37 (±0,08) (valor p <0,05). Tras la transformación logarítmica, los análisis multivariables lineales generales revelaron una diferencia significativa de grosor íntima-media carotídeo en pacientes con síndrome nefrótico (valor p <0,001). Posteriormente, el factor que influyó sobre la TMIR fue la duración de la enfermedad (p <0,05).
Conclusiones: La mitad de los niños con síndrome nefrótico a los que se les realizó una ecocardiografía presentó hipertrofia ventricular izquierda. Se correlacionó con la rigidez carotídea y la hipertensión sistólica.
Palabras clave: Síndrome nefrótico idiopático, Niño, Hipertrofia ventricular izquierda, Grosor íntima-media carotídeo.
Introduction
Carotid intima-media thickness (cIMT) is an indirect marker of atherosclerosis and target organ damage in adults. Its value in children is still under debate but there are increasing numbers of studies among children with risk factor for vascular damage.1A recent systematic review has reported moderate correlation between cIMT and coronary atherosclerosis or cardiovascular events.2,3 Hemodynamic, metabolic, and inflammatory factors change reversibly the arterial wall thickness. Impaired function of endothelium has been shown in acute phase of idiopathic nephrotic syndrome.4 Nephrotic patients are prone to myocardial infarction and coronary artery disease secondary to hypoalbuminemia, dyslipidemia, and hypercoagulopathy state.5,6
Few studies of cIMT in nephrotic children show to be correlated with duration of disease and unresponsiveness to steroid; however, none of them investigates carotid function including distensibility, stiffness, and elasticity.7,8 We evaluated carotid function as an atherosclerosis surrogate marker in children with nephrotic syndrome.
Methods
Between 2008 and 2011, all children with history of idiopathic nephrotic syndrome were enrolled in the study. The inclusion criteria were: 1- Idiopathic nephrotic syndrome with normal serum complement, 2- Being on therapy (continuous or interrupted) for at least one year, 3- Glomerular filtration rate more than 20mL/min/1.73m2, 3-Age above two years at the time of study, 4- Absence of acute infection in previous three months, 5- Absence of severe edema at the time of investigation. The control group consisted of 79 age-sex-matched children with history of urinary tract infection who attended outpatient clinic. Informed consent was obtained from parents or patients. Weight, height, and blood pressure were recorded. Past medical and drug history were taken. Hypertension was defined as having systolic or diastolic blood pressure equal or more than 95% for age, height, and sex. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki revised in Tokyo 2008. It was approved by ethical committee of Iran University of medical sciences and followed by the institution's Review Board for Human Subjects guidelines.
Patients were classified according to the following definitions of nephrotic syndrome: Steroid response was defined as to achieve complete remission after four weeks of steroid therapy. The lack of remission despite of one month steroid therapy and three consecutive pulses of methylprednisolon considered as steroid resistant. Two subsequent relapses during steroid therapy or within two weeks after discontinuation of therapy categorized as steroid dependent. One-year follow- up was required to discriminate between frequent (more than 3 relapses within a year) and infrequentrelapses.9
Carotid intima-media thickness (cIMT) was measured as the following way:
The intima-media thickness (IMT) of the common carotid artery (CCA) on each side was measured using a GE Logic 500 sonography system with two 7.5MHz linear and 5MHz convex probes.
The IMT is defined as the distance between the first (lumen-intima interface) and the second (media-adventitia interface) echogenic line of the far wall, using a manual cursor placement measurement technique. The measurement was performed at a point 10-20mm proximal to the common carotid bifurcation at a 10mm distance of far wall, at systolic phase of cardiac contraction (maximum diameter of the common carotid artery (CCA) lumen), in mid-longitudinal plane using maximum acceptable magnification of the image of the far wall. The examination was performed at supine position with a slightly overextended neck after 10 minutes of rest. Two measurements were made on each side, and the mean value was calculated separately for each CCA. The internal diameter of CCA was also measured at the same site at both systolic (maximum diameter) and diastolic (minimum diameter) phases in axial image with maximum acceptable magnification by which both near and far wall of the CCA could be seen clearly. Only images accepted as of good quality were taken to further analysis.
The radiologist was not aware of the clinical data of the patients or control group.
The following parameters were calculated according to formulas described by Aggoun et al.10 and Tounian et al.11:
- Mean systolic diameter (sD): (LsD+RsD)/2, where LsD is the left CCA systolic diameter and RsD is the right CCA systolic diameter;
- Mean diastolic diameter (dD): (RdD+LdD)/2, where RdD is the right CCA diastolic diameter and LdD is the left CCA diastolic diameter;
- Mean lumen cross sectional area of the artery (LCSA); π(dD)2/4
- Mean wall cross-sectional area (WCSA);π(dD/2-IMT)2-π(dD/2)2
- Mean cross-sectional distensibility coefficient (DC) =2(∆D/dD)/∆P, where ∆D is difference between systolic and diastolic diameter of common carotid artery.
- Arterial stiffness coefficient β (β): (ln [SBP/DBP])/(∆D/D)
- Incremental elastic modulus (Einc): [3(1+LCSA/WCSA)]/DC
Echocardiography examination was performed through standard transthoracic echocardiography using a Vivid 3 (General Electric Medical Systems, Horten, Norway, 2.5-7MHZ probes) equipped with tissue Doppler technology as previously described.12 All measurements were carried out by one examiner who was blinded to the status of the subjects. Left ventricular mass (LVM) was assessed by using two dimensional directed M-mode echocardiography. Then, LVM index was calculated to evaluate left ventricular hypertrophy (LVH). LVM Index more than 38g/H2.7was defined as LVH.
Plasma creatinine was measured by Jaffe method using creatinin kit (Pars Azmun) Biolis 24 I Premium device (Tokyo boeki medical system). Glomerular filtration rate (GFR) was calculated based on serum creatinine by using Schwartz formula [K×Height (cm)/Pcr].13 GFR more than 90ml/min/1.73m2 considered normal.
Children with history of urinary tract infection with normal renal function on imaging study, normal blood pressure, normal glomerular filtration rate, and no history of early atherosclerosis in family history entered into the study as controls. The Student-t-test was used to compare means, General Linear Univariate Model of variance and covariance used for correlation between variables. The analysis performed on variables both in categorical and continuous forms. The variables that had not normal distribution, log transformation was performed before analysis. Because of small sample size, Multivariate analysis performed between carotid parameters and those independent variables that showed significant correlation by Univariate analysis. P<.05 was considered significant.
Results
From 55 children who enrolled into the study, 18 (32.7%) were resistant to steroid, 19 of them were steroid dependent, and 18 (32.7%) were responsive (Table 1). Only 15 cases had renal biopsy including focal segmental glomerulosclerosis (n=12) and minimal change nephrotic syndrome (n=3). At the time of participation, 27 of patients were in remission (49%). Frequent relapses were found in 27 cases (49%).
Table 1. Demographic data of children with nephrotic syndrome and controls 
ACEI: Angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, BMI: Body mass index,
cm: centimeter, DBP: diastolic blood pressure, GFR: glomerular filtration rate, HDL: High density lipoprotein,
Kg: kilogram, N: number, SBP: systolic blood pressure, SD: standard deviation, y: year.
aChi-square test, b non-parametric median test.
From 134 participants, seven subjects (4 cases, 3 controls) were excluded due to lack of carotid measurements. Table 2 depicted carotid parameters in both nephrotic patients and in controls. Mean cIMT and WCSA were significantly higher in nephrotic children than controls. We evaluated the effect of gender, age, GFR, BMI, blood pressure, proteinuria, lipid profile, and disease statues on carotid measurements (Table 3). As shown in table 3, hypertension had influence on distensibility, stiffness, carotid remodeling, and LCSA. Multivariate analysis revealed correlation between mCIMT and nephrotic (p-value=.029). Moreover, there was correlation between age and LCSA (p-value=.007) or WCSA (p-value=.036).
Table 2. Demographic data of children with nephrotic syndrome and controls 
DC: cross-sectional distensibility coefficient, dD: Mean diastolic diameter, Einc: Incremental elastic modulus,
LCSA: lumen cross sectional area of the artery, mCIMT: mean carotid intima media thickness,
mLCC: mean left carotid intima media thickness, mRCC: mean right carotid intima media thickness,
sD: Mean systolic diameter, WCSA: wall cross-sectional area, β: Arterial stiffness coefficient.
Table 3. General Linear univariate model for analyzing the effect of Group, BMI,
Blood pressure, gender, age, and proteinuria on carotid parameters 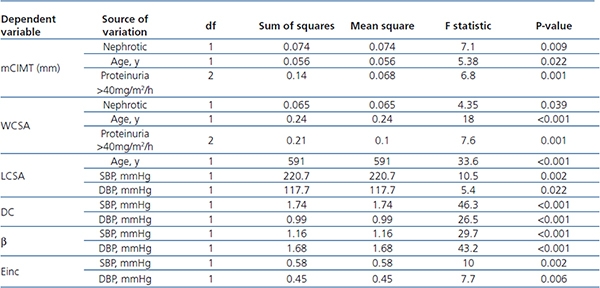
Β: Arterial stiffness coefficient, DBP: Diastolic blood pressure, DC: cross-sectional distensibility coefficient,
Einc: Incremental elastic modulus, LCSA: lumen cross sectional area of the artery, mCIMT: mean carotid
intima media thickness, SBP: Systolic blood pressure, WCSA: wall cross-sectional area, Y: year.
Subsequently, nephrotic children were classified to steroid sensitive, dependent, and resistant. Mean cIMT and carotid parameters were not statistically different in three groups (P>.05). Subgroup analysis performed for lipid profile, serum albumin level, GFR, age, duration of disease, hypertension, BMI, gender, steroid response, relapse statues, immunosuppressive regimen, and carotid parameters. cIMT was correlated with duration of disease more than 2 years (p-value=.04, 95% CI: 0.002-0.12). Even after excluding children with GFR less than 90mL/min/1.73m2, this correlation remained statistically significant (p-value =.031).
Echogradiographic measurement showed LVH in one (1.9%) out of 53 healthy children and 19 (50%) out of 38 children with nephrotic syndrome (p-value<.001). There was correlation between LVH and carotid stiffness (p-value=.005), nephrotic group (p-value<.001), proteinuria (p-value<.001), and systolic hypertension (p-value=.019). Multivariate analysis revealed LVMI was affected mainly by hypertension (p-value=.004). This observation showed early changes of carotid parameters in nephrotic children. It seems that changes of carotid parameters occurred earlier than compensatory LVH to be appeared.
Discussion
Despite the similarity of both groups in basic demographic data, carotid intima-media thickness and WCSA were significantly thicker in nephrotic patients .cIMT in nephrotic children was mainly correlated with duration of disease. There was no correlation between cIMT and LVH per se. There are few published studies about the value of cIMT in nephrotic children. Leno C et al. reported on an extensive myocardial infarction in a 7-year old with minimal change nephrotic syndrome and resistant to corticosteroid. Diagnostic angiography confirmed the presence of atherosclerotic plague in right coronary artery and circumflex artery. Concomitant dyslipidemia with a trait of familial hyperlipidemia considered as risk factors for atherosclerosis.5 The levels of total cholesterol, LDL, homocysteine, apoprotein B and A1 were significantly higher in children with history of idiopathic nephrotic syndrome being off treatment for 4 to 15 years.7 Mean cIMT was not different in previously treated idiopathic nephrotic syndrome and controls. But multifactorial analysis revealed strong correlation between IMT and the rate of recurrence of the disease.7 The thicker cIMT was correlated with severity of proteinuria that reflected the effects of tissue edema. In our study, hypertension and duration of disease were the main independent variables that significantly correlated with carotid parameters. Bussy et al. evaluated the effective vs. intrinsic stiffness in 104 hypertensive and 40 normotensive patients. Effective stiffness was significantly higher in hypertensive subjects.14 We found a correlation between carotid stiffness or distensibility and high blood pressure. A meta-analysis about the effect of antihypertensive treatment on carotid intima-media thickness include a large number of patients treated with antihypertensive, placebo, or diuretics revealed the beneficial effect of usage of antihypertensive on cIMT.3 In our study, there was no correlation between cIMT and treatment with angiotensin converting enzyme inhibitors or angiotensin receptor antagonists.
cIMT is a surrogate marker for evaluation of diffuse thickening and stiffening of large and medium sized vessels. It is secondary to hypertension and atherosclerosis that is focal lesion secondary to hyperlipidemia and metabolic disorder. Cardiovascular disease preceded by endothelial dysfunction and cIMT changes.4 A systematic review by Lorenz MW et al. questioned about the correlation between cIMT, stroke, and myocardial infarction on 200000 patients. They found hazard risk of 1.15 for stroke and hazard ratio of 1.18 for myocardial infarction per 0.1mm change of carotid thickness.2 In our study, the mean cIMT and WCSA were thicker in nephrotic children. Subgroups analysis revealed that duration of the disease and hypertension were the main variables correlated with carotid parameters. This finding indirectly showed the vascular changes in nephrotic patients. Our study showed that episodes of hypertension contributed to these vascular changes and left ventricular hypertrophy. Carotid changes occur earlier than left ventricular hypertrophy. Hence, it can be an easy tool for early assessment of vascular changes before end organ damage such as LVH happen. Previous investigation revealed the reversibility of carotid changes; therefore, serial assessment of carotid parameters, blood pressure, and lipid profile are necessary in nephrotic patients.
It was interesting that significant number of children with nephrotic syndrome had abnormal left ventricular mass index and it was more frequent in those children with systolic hypertension and had carotid stiffness. The severe chronic kidney disease was not entered into the study; therefore, LVH was found in earlier stage of CKD in nephrotic patients. Our previous study showed higher rate of progression to end stage of renal disease in steroid resistant nephrotic syndrome despite of receiving various immunosuppressive medication.15-18 We analyzed the steroid resistant children as a separate group and compared with the rest of cases and could not find any differences. Moreover, there was no correlation with immunosuppressive and carotid parameters. By excluding children with GFR less than 90mL/min/1.73m2, 41.7% of patients had LVH. Tafreshi et al. showed the superiority of tissue Doppler over conventional method to detect early myocardial performance index in children with mild to moderate chronic kidney disease.12 Simpson et al. studied different methods of left ventricular mass indexation on 85 patients with chronic kidney disease. They found a discrepancy on the categorization of LVH. The rate of LVH was the lowest by z-score indexed by body surface area (18%) to the highest rate of 55% by indexation of LVM to height power of 2.7. This wide range has a large impact on treatment.19 Brumback et al. measured left ventricular mass by cardiovascular magnetic resonance and developed a new allometric index. The same differences in the prevalence of hypertrophy were detected between indices of LVM to weight, height, non indexation, and new allometric method.20
In a case -control study, Qin et al. evaluated right ventricular function in 50 nephrotic children in relapse or initiation of disease. They found increase in right ventricular end diastolic pressure and decrease in right ventricular ejection fraction in nephrotic group. The right ventricular dysfunction was correlated with length of disease and TNF-α. The explanation was the occurrence of subclinical pulmonary microthrombosis that led to pulmonary hypertension.21 Le et al. measured cIMT and atherosclerosis promoting risk factors in 70 children with obesity or familial dyslipidemia. They studied the prevalence of advance vascular age and its correlation with multiple risk factors including hyperlipidemia, insulin resistant statues, smoking, and hypertension. They used subclinical atherosclerosis definition for classifying advanced vascular age in children (maximum cIMT>=25% sex-match 45-years adult). The prevalence of advanced vascular age was about 73-75% in both groups. As we know, nephrotic children have a trend to hyperlipidemia, obesity, hypertension, or thromboembolic events. In our study, the prevalence of advanced vascular age was significantly higher in nephrotic children (29.4% vs. 10.7, p-value=.008). Length of disease more than two years was more associated with advanced vascular age (48% vs.11.5%, p-value=.006). It would be a major concern for adults with childhood history of nephrotic syndrome. They might be more prone to atherosclerosis and vascular changes. Accordingly, longer follow up even during adulthood, close observation, change of life style, modifying atherosclerosis risk factors might be consider seriously.
The limitations of this study were absence of longitudinal assesses of cIMT in nephrotic children and low power of the study. Although cIMT was assessed before increment of steroid dosage, any changes of immunosuppressant, and when the child had no severe edema during relapse phase, the effect of hemodynamic changes could not be ignored.
Conclusions
cIMT was thicker in nephrotic children. The other carotid parameters were correlated with hypertension and longer duration of disease. Left ventricular hypertrophy was more frequent in nephrotic children and correlated with carotid stiffness and systolic hypertension. Carotid parameters changes occurred earlier than compensatory LVH to be appeared.
Conflicts of interest
Authors declare potential Conflicts of Interest.
References
1. Litwin M, Niemirska A. Intima-media thickness measurements in children with cardiovascular risk factors. Pediatr Nephrol 2009;24:707-19. [ Links ]
2. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115:459-67. [ Links ]
3. Wang JG, Staessen JA, Li Y, Van Bortel LM, Nawrot T, Fagard R, et al. Carotid intima-media thickness and antihypertensive treatment: a meta-analysis of randomized controlled trials. Stroke 2006;37:1933-40. [ Links ]
4. Tkaczyk M, Czupryniak A, Owczarek D, Lukamowicz J, Nowicki M. Markers of endothelial dysfunction in children with idiopathicnephrotic syndrome. Am J Nephrol 2008;28:197-202. [ Links ]
5. Leno C, Pascual J, Polo JM, Berciano J, Sedano C. Nephrotic syndrome, accelerated atherosclerosis, and stroke. Stroke 1992;23:921-2. [ Links ]
6. Hopp L, Gilboa N, Kurland G, Weichler N, Orchard TJ. Acute myocardial infarction in a young boy with nephrotic syndrome: a case report and review of the literature. Pediatr Nephrol 1994;8:290-4. [ Links ]
7. Kniazewska MH, Obuchowicz AK, Wielkoszyński T, Zmudzińska-Kitczak J, Urban K, Marek M, et al. Atherosclerosis risk factors in young patients formerly treated for idiopathic nephrotic syndrome. Pediatr Nephrol 2009;24:549-54. [ Links ]
8. Ksiazek J, Niemirska A, Lipka M, Grenda R. Evaluation of arterial intima-media thickness (IMT) in children with idiopathic nephrotic syndrome-preliminary report. Przegl Lek 2006;63:205-7. [ Links ]
9. Niaudet P, Boyer O. Idiopathic nephrotic syndrome in children: clinical aspects. In: Avner ED, Harmon WE, Niaudet P, Yoshikawa N (eds.). Pediatric Nephrology. Berlin Heidelberg: Springer-Verlag; 2009. p. 667-702. [ Links ]
10. Aggoun Y, Sidi D, Levy BI, Lyonnet S, Kachaner J, Bonnet D. Mechanical properties of the common carotid artery in Williams syndrome. Heart 2000;84:290-3. [ Links ]
11. Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, et al. Presence of increased stiffness of the common carotid artery and enodethelial dysfunction in severely obese children: a prospective study. Lancet 2001;358:1400-4. [ Links ]
12. Tafreshi RI, Human N, Otukesh H, Nikavar A. Evaluation of combined left ventricular function using the myocardial performance index in children with chronic kidney disease. Echocardiography 2011;28:97-103. [ Links ]
13. Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 1987;34:571-90. [ Links ]
14. Bussy C, Boutouyrie P, Lacolley P, Challande P, Laurent S. Intrinsic stiffness of the carotid arterial wall material in essential hypertensives. Hypertension 2000;35:1049-54. [ Links ]
15. Otukesh H, Otukesh S, Mojtahedzadeh M, Hoseini R, Fereshtehnejad SM, Riahi Fard A, et al. Management and outcome of steroid-resistantnephrotic syndromein children. Iran J Kidney Dis 2009;3:210-7. [ Links ]
16. Hoseini R, Otukesh H, Fereshtehnejad SM, Tahoori A, Hooman N, Rahimzadeh N, et al. Prevalence and outcome of focal segmental glomerulosclerosis in Iranian children withnephrotic syndrome. Iran J Kidney Dis 2012;6:18-24. [ Links ]
17. Nickavar A, Safarzadeh AE, Sotoudeh K, Otukesh H, Hooman N. Mycophenolate mofetil for treatment of idiopathic nephrotic syndrome in children. Iran J Kidney Dis 2012;6:346-9. [ Links ]
18. Hooman N, Esfehani ST, Madani A, Bodagi E, Mohseni P. Clinicopathologic features and outcomes of membranous nephropathy in Markaz tebi children hospital. Iranian Journal of Pathology 2006;1:69-74. [ Links ]
19. Simpson JM, Savis A, Rawlins D, Qureshi S, Sinha MD. Incidence of left ventricular hypertrophy in children with kidney disease: impact of method of indexation of left ventricular mass. Eur J Echocardiogr 2010;11:271-7. [ Links ]
20. Brumback LC, Kronmal R, Heckbert SR, Ni H, Hundley WG, Lima JA, et al. Body size adjustments for left ventricular mass by cardiovascular magnetic resonance and their impact on left ventricular hypertrophy classification. Int J Cardiovasc Imaging 2010;26:459-68. [ Links ]
21. Hooman N, Mostafavi SH, Hallaji F, Isa-Tafreshi R, Otukesh H. The Correlation between Atherosclerosis Risk Factors and Carotid Intima Media Thickness in Children with Nephrotic Syndrome. Pediatr Nephrol 2010;25:1957. [ Links ]
![]() Correspondence:
Correspondence:
Nakysa Hooman,
Pediatric Nephrology,
Pediatric Transplantation and Dialysis Research Center (PTDRC),
Ali-Asghar children hospital,
Iran University of Medical Sciences,
Vahid Dastgerdi, 1919816766, Tehran, Iran
nhooman@tums.ac.ir
nakisa45@yahoo.com
Enviado a Revisar: 26 Mar. 2013
Aceptado el: 18 May. 2013














