Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nefrología (Madrid)
versión On-line ISSN 1989-2284versión impresa ISSN 0211-6995
Nefrología (Madr.) vol.34 no.1 Cantabria 2014
https://dx.doi.org/10.3265/Nefrologia.pre2013.Oct.12234
ORIGINALES BREVES
The First Report of The Latin American Society of Nephrology and Hypertension (SLANH) Anemia Committee in Chronic Hemodialysis Patients
Primer Informe del Comité de Anemia en Pacientes en Hemodiálisis Crónica de la Sociedad Latinoamericana de Nefrología e Hipertensión (SLANH)
Raúl Carlini1, Gregorio Obrador2, Nieves Campistrús3, Liliana Andrade4, Liliana Chifflet5, Rachel Bregman6, Alberto Locatelli7, Ricardo Correa-Rotter8 and Hugo Poblete9
1Division of Nephrology. University Hospital of Caracas. Caracas (Venezuela)
2School of Medicine. Panamerican University. Mexico City (Mexico)
3Dialysis Center Uruguayana. Montevideo (Uruguay)
4Hospital Policial Medical Complex Churruca-Visca. Buenos Aires (Argentina)
5Division of Dialysis. San José Medical Association. San José de Mayo (Uruguay)
6Rio de Janeiro State University. Rio de Janeiro (Brazil)
7Nefronosa Service SA. Buenos Aires (Argentina)
8Department of Nephrology and Mineral Metabolism. Institute of Nutrition, Salvador Zubirán. Mexico City (Mexico)
9Dialysis Center Sermedial. Valparaíso (Chile)
ABSTRACT
Background: Anemia almost invariably occurs in patients with chronic kidney disease. Limited data are available regarding anemia management in Latin American (LA) hemodialysis (HD) patients.
Objective: To evaluate the results of the first anemia survey of the Anemia Committee of the SLANH.
Methods: This is a multinational, voluntary survey that collected anemia management data from adult HD patients from independent, non-chain owned HD units, between 09/2009 and 03/2010. T-test, ANOVA, chi-square test and multivariate logistic regression were used for statistical analysis.
Results: The survey received responses from 134 HD units of 16 countries providing data from 9,025 patients. Mean values of Hb, ferritin, and transferrin saturation (TSAT) were 10.5±1.8g/dL, 570±539μg/l, and 29.8±15%, respectively. Only 32.7% of patients were within the Hb target of 10.5-12.0g/dL (46.3% were below and 21.1% above). Erythropoietin-stimulating agents (ESAs) were administered to 84.3% patients and 68.3% received intravenous iron (IV). Iron deficiency (TSAT≤20%) was present in 27.5% patients and among those receiving erythropoietin, 47% did not achieve Hb target. The independent variables associated with the lowest Hb level (<10.5g/dL) were: female gender, TSAT<25% and age<50 years.
Conclusions: According to these results, nearly half of LA chronic HD patients did not achieve the recommended Hb target despite wide use of ESAs and IV iron.
Key words: Anemia, Hemodialysis, Survey, Latin America.
RESUMEN
Antecedentes: La anemia representa una complicación frecuente en pacientes con enfermedad renal crónica. En Latinoamérica (LA) la prevalencia y características de la anemia en pacientes en hemodiálisis (HD) no ha sido bien estudiada.
Objetivo: Evaluar los resultados del primer registro de anemia del Comité de Anemia de la Sociedad Latinoamericana de Nefrología e Hipertensión.
Métodos: Esta es una encuesta multinacional, voluntaria, que recolectó datos sobre el tratamiento de la anemia en pacientes en HD de unidades independientes en LA entre septiembre de 2009 y marzo de 2010. Para el análisis estadístico se utilizaron los siguientes métodos: t-test, ANOVA, χ2 y el análisis de regresión logística multivariante.
Resultados: La encuesta fue respondida por 134 unidades de HD de 16 países, recibiéndose datos de 9025 pacientes. Las medias ± desviación estándar de Hb, ferritina y del índice de saturación de transferrina (IST) fueron respectivamente: 10,5 ± 1,8 g/dl, 570 ± 539 μg/l y 29,8 % ± 15. Se administró agentes estimulantes de la eritropoyesis (AEE) al 84,3 % de los pacientes y hierro intravenoso (FeIV) al 68,3 %. Solamente el 32,7 % de los pacientes tuvieron una Hb blanco de 10,5-12,0 g/dl. En pacientes con Hb < 10,5 g/dl, el 85,2 % estaban recibiendo AEE y un 68 % FeIV. Las variables independientes asociadas con nivel Hb < 10,5 g/dl fueron: género femenino, IST < 25 % y edad < 50 años. En la muestra de pacientes analizada, a pesar del amplio uso de los AEE y FeIV, casi la mitad de los pacientes no alcanzó la Hb blanco.
Palabras clave: Anemia, Hemodiálisis, Encuesta, Latinoamérica.
Introduction
Anemia is an important and almost universal complication of advanced chronic kidney disease (CKD). Since the introduction of recombinant human erythropoietin in 1989, several studies have reported that erythropoietin-stimulating agents (ESAs) increase hemoglobin (Hb) levels, improve quality of life, and reduce requirements for blood transfusion. However, the therapeutic Hb target remains a controversial issue. Three studies in predialysis patients (Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease (CHOIR), Normalization of Hemoglobin Level in Patients with Chronic Kidney Disease and Anemia (CREATE), and A Trial of Darbepoetin Alfa in Type 2 Diabetes and Chronic Kidney Disease (TREAT) reported that normalization of Hb was associated with no benefit in terms of cardiovascular morbidity and mortality, and with an increased risk of stroke and cancer in patients with a history or current malignancy.1-3 A recent study by Coyne demonstrated that in HD patients with ischemic heart disease or heart failure, higher Hb (13-15g/dL) could increase nonfatal myocardial infarcts, deaths, and has no effect in improving quality of life compared to those with Hb of 11g/dL.4
Since 1997, several clinical practice guidelines for the management of anemia of CKD have been published. The 2007 version of the National Kidney Foundation-Kidney Disease Quality Outcomes Initiative (NKF-KDOQI) suggested a Hb target between 11 and 12g/dL, while avoiding levels above 13g/dL.5 Two years later, the Anemia Working Group of European Renal Best Practice (ERBP) endorsed these recommendations.6 In 2008, the Anemia Working Group of the Latin American Society of Nephrology and Hypertension (SLANH) (RPCA-ERC/SLANH) recommended to maintain Hb levels between 10.5 and 12g/dL, ferritin levels between 200 and 500μg/L and transferrin saturation (TSAT) levels between 30 and 40%.7 Finally, the recently published Kidney Disease Improving Global Outcomes (KDIGO) anemia guidelines suggested to consider starting ESAs therapy in iron replete HD patients when the Hb levels are between 9.0 and 10.0g/dL, and to maintain it at ≤11.5g/dL. It also recommended to individualize therapy by taking into consideration the balance between benefits of reducing blood transfusions and anemia-related symptoms and risks associated with ESAs therapy.8
In the Latin American (LA) region, little is known about anemia management in dialysis patients. Interestingly, LA patient populations consist of a variety of races that live anywhere from sea level to mountainous regions with altitudes as high as 13,000 feet. Furthermore, there are important socioeconomic disparities and health care systems that vary from country to country. These and other factors may influence anemia management in CKD patients resulting in variations in Hb levels.
The Latin American Society of Nephrology and Hypertension (SLANH) survey collected data of patients receiving kidney replacement therapy in 20 LA countries. In its latest report, 147,158 patients were receiving chronic HD in the region.9 However, scant anemia management data are available. Therefore, under the auspices of the SLANH, the Latin American Anemia Committee was established to specifically address this issue.
In this paper, we present the first report of the SLANH's anemia survey with data on 9,025 HD patients from 16 LA countries.
Methods
In this cross-sectional study, a survey developed by the Anemia Committee was sent to SLANH's affiliated national societies of nephrology and was then distributed to the different local dialysis units who were independent, non-chain owned. Nephrologists were requested to fill out the survey with anemia management data collected between September and December 2009, although this period had to be extended through March 2010.
Inclusion criteria were adult (≥18 years of age) patients receiving chronic HD for at least 3 months. Data collected included age, gender, cause of CKD, dry weight, last Kt/V, time on HD, type of ESA used, total weekly dose, route of administration [intravenous (IV) or subcutaneous (SC)], type of IV iron preparation used, total monthly dose, last Hb, ferritin, and TSAT (during the data collection period), and blood transfusion use during the prior two months.
Databases of each country were validated and merged into a single database. Missing and out-of-range values were excluded. Acceptable ranges were as follows: Hb: 3-16g/dL, ferritin: <5,000μg/L, TSAT: 5-100%, time on HD: 0.3-28 years, Kt/V: 0.5-3.5, weekly ESA dose (erythropoietin-α or β): 1,000-30,000IU, and monthly IV iron dose: 50-2,400mg. Selection of these ranges was based on the distribution of data and clinical judgment. Descriptive statistics were used to analyze patient and anemia management data. T-test or ANOVA were used for continuous variables and chi square test for categorical variables. A p-value of <0.05 was considered statistically significant. For some analyses, patients were stratified according to Hb level <10.5g/dL (low), 10.5-12.0g/dL (target range), and >12.0g/dL (high). Multivariate conditional logistic regression analysis was performed to determine factors associated with Hb≥10.5g/dL versus ≤10.5g/dL.
Independent variables were entered by the stepwise forward method and included age, gender, ferritin, TSAT and Kt/V levels. All statistical analyses were performed with the Statistical Package for Social Sciences SPSS, version 17, Chicago, IL.
Results
Baseline patient characteristics
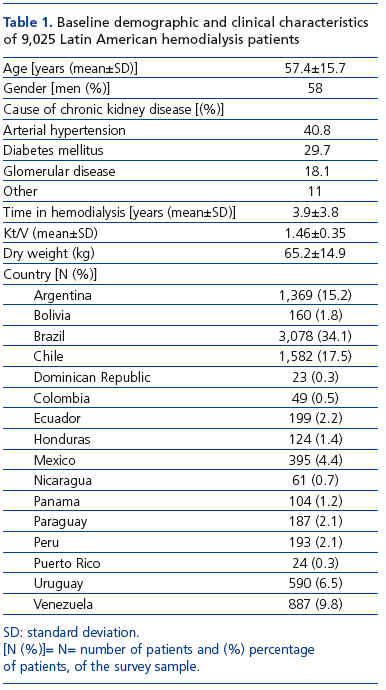
A total of 171 surveys from the same number of unit hemodialysis centers were received. From those surveys, 134 had all inclusion criteria and a total of 9,025 chronic HD patients from 16 LA countries were incorporated in the analysis. Baseline patient characteristics are shown in Table 1. Mean age of the patients was 57.4±15.7 years and 58% were men. Causes of CKD were arterial hypertension (40.8%), diabetes mellitus (29.7%), glomerular disease (18.1%) and other causes (11%). Mean time in HD was 3.9±3.8 years. Brazil, Chile, and Argentina accounted for two-thirds of the patients (34.1%, 17.5%, and 15.2%, respectively).
Anemia management
Results of anemia management are shown in Table 2. The majority of patients (84.7%) were receiving some type of ESA during the study period. Erythropoietin-α and erythropoietin-β were used in 73% and 10.1% of the patients. The median weekly dose for erythropoietin-α was 6,000IU with SC administration and 11,000IU with IV administration and the corresponding average SC weekly doses were 7,330±4,276UI/week or (118±75UI/Kg/week) and 10,622±6,072UI/week (171±104UI/Kg/week), respectively (p=0.0001). ESAs were more often administered by the SC route (83.9%).
About two thirds (68.3%) of the patients were receiving IV iron. Iron saccharate and low molecular weight iron dextran were used in 56.3% and 10% of patients, respectively. The median iron monthly dose for both iron preparations was 200mg, whereas the mean monthly doses for iron sacharate were 317±243mg (range 50-1,400mg/month) and for the low molecular iron weight iron dextran 318±230mg (range 50-1,000mg/month). No significant differences in mean iron doses were observed between male and females, or between patients with or without diabetes. Blood transfusions in the prior two months were given to 8.2% of patients.
Hemoglobin and iron values
Mean (±SD) values of Hb (n=9,025), ferritin (n=7,915), and TSAT (n=5,705) were 10.5±1.8g/L, 570±539mg/L, and 29.8±15%, respectively. Most of the women (52%) had Hb≤10.5g/dL, meanwhile 58% of the men had Hb≥10.5g/dL (p=0.0001). However, ESA doses were higher in women (137U/Kg/week) than in men (116U/Kg/week), (p=0.0001).
Only 32.7% of patients were within the Hb target recommended by the RPCA-ERC/SLANH, the remaining 46.3% and 21.1% of patients had Hb levels below (<10.5g/dL) or above (>12.0g/dL) the recommended target, respectively. Figure 1 shows the distribution of Hb levels by the participating countries. Chile, Paraguay and Venezuela were the countries with the lowest mean Hb values (<10.5g/dL).
Factors associated with hemoglobin levels
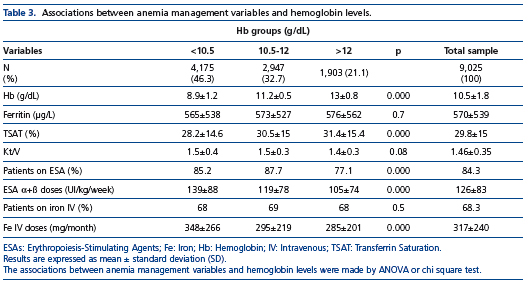
Table 3 depicts univariate associations between several anemia management variables and Hb levels within and outside the recommended target. A higher TSAT was associated with
the highest Hb levels. Additionally, higher ESA and IV iron doses were associated with Hb levels below the recommended target. Among patients with Hb level between 10.5-12g/dL, 62.4% had TSAT≥25%, whereas only 46.6% of those with Hb levels <10.5g/dL had a TSAT≤25% (p=0.0001). Moreover, in the group with Hb<10.5g/dL, the ESAs were prescribed in 84,3% and iron IV in 68.3% of the patients. Further variables, such as ferritin and Kt/V, were not significantly different among the Hb groups. The results of a multivariate logistic regression analysis demonstrated that the variables independently associated with Hb levels <10.5g/dL were: age≤50 years (RR=1.17, 95%CI=1.04-1.32, p<0.01), female (RR=1.37, 95%CI=1.22-1.54, p<0.001), and TSAT≤25% (RR=1.43, 95%CI=1.27-1.6, p<0.0001) (data not shown).
Discussion
The main findings of this first report of the SLANH's anemia survey can be summarized as follows: a) the majority of patients attending independent, not-chain owned chronic HD facilities were receiving an ESA; b) erythropoietin α was more frequently used, at a median weekly dose of 6,000IU SC and 11,000IU IV; c) SC was the preferred route of ESA administration; d) two-thirds of patients were receiving IV iron; e) mean values of Hb, ferritin, and TSAT were 10.5±1.8g/dL, 570±539μg/L, and 29.8%±15%, respectively; f) only one-third of patients were within the SLANH's recommended Hb target of 10.5-12.0g/dL, and nearly half of patients had Hb values below 10.5g/dL; g) age≤50 years, female, and TSAT≤25% were independently associated with having a Hb level below the recommended target.
Published anemia data in the management of chronic HD patients in LA is scarce. Despite important limitations, possible sources of information are individual Register from local LA nephrological societies. The 2008 Brazilian Society of Nephrology's Dialysis Census Report reported that 83% of chronic HD patients were receiving an ESA and 53.5% an iron preparation.10 Additionally, 47.7% had Hb levels <11g/dL. Another study of 32,136 Brazilian chronic HD patients reported that being male and having an age of <65 years, diabetic CKD, or an arteriovenous fistula, were independently associated with ESAs use.11
The 2009 Uruguayan Society of Nephrology Register reported that 94% of chronic HD patients were receiving an ESA and 65% iron; it also reported that 62.3% of patients and 19.5% had Hb levels between 10.5-12g/dL and <10g/dL, respectively.12 The 2010 Register from the Chilean Society of Nephrology reported that, among 14,462 chronic HD patients, 54.6% had a hematocrit <30%, and that 42.5% were receiving ESA treatment and 51.7% IV iron.13 Overall, these data demonstrate that there is significant variability in anemia management among the different LA countries.
Comparison of our results with those of other non-LA countries is difficult to assess in part due to variations of Hb target levels in chronic HD patients in different clinical practice guidelines.8,14,15 This SLANH's anemia survey is a first attempt to try to elucidate the status of anemia management among LA chronic HD patients. We found that the median Hb level was 10.5g/dL and that only 34% of patients had Hb levels within the target recommended by the RPCA-ERC/SLANH. However, a subsequent statistical analysis showed that 61.4% of patients had Hb levels above 10g/dL. Surprisingly, 84% of the patients were receiving an ESA and 68% IV iron treatment.
Regarding ESA doses, the mean SC dose of erythropoietin α was 7,330IU/week and in the IV administration was 10,662IU/week to achieve median Hb levels of 10.5g/dL in slightly over one-third of patients. Most other Society Registers do not report the discrimination between IV and SC ESAs doses. The 2010 UK Annual Renal Register reported a mean ESA dose of 9,507IU/week and that 85% of patients had Hb levels ≥10g/dL, with the median level being 11.6g/dL.16 The 2012 US Renal Data Report shows that by the end of 2010, the mean ESA dose was 15,829IU/week with an achieved mean Hb level of 11.3g/dL. Roughly, two-thirds of patients had Hb levels in the 10-12g/dL range. Additionally, the highest doses of IV iron of 2,700 mg or more were present in 24% of patients who were treated jointly with ESAs.17
In a recent report from Japan, of 1,622 patients the mean Hb was 10.3g/dL. The majority were treated with ESAs (82.2%), but only 54% of the patients had Hb levels within the target recommended by the Japanese guidelines. The mean ESAs doses administered by IV were 4,645IU/week.18
Our findings also demonstrate that the RPCA-ERC/SLANH recommended levels of TSAT and ferritin (>25% and ≥200μg/L, respectively) were associated with the highest Hb levels. We also found a negative association between ferritin and TSAT with ESAs and iron IV doses. The association between the low Hb and TSAT≤25% suggests that a functional iron deficiency could be present in a high percentage of patients. However, the low percentage of patients that were tested for TSAT (63%) suggests that the assessment for a complete iron status testing is necessary for the adequate control of anemia in HD patients in LA. The mechanism of poor anemia control detected in women, even though receiving higher weight adjusted ESA doses, was probably due to the fact that women were iron undertreated when compared to men (47% vs 52%, respectively).
We also found that ESAs and iron IV doses were higher for patients with low Hb levels which could suggest ESA hyporesponsiveness. Multivariable logistic regression analysis demonstrated that the independent variables associated with Hb level <10.5g/dL were female gender, TSAT≤25%, and age≤50 years. Female gender and low TSAT are known as factors for ESA hyporesponsiveness.19,20 The variable age≤50 year is probably because our HD population is younger than that reported in other populations.
The inter-variation in the Hb levels between LA countries could be due to several causes, such as anemia treatment (including laboratory test and drugs) which represents an important cost for the diverse health care systems. This could reflect a potential restriction in anemia management practices, not only for the prescription of inappropriate doses, but also for the frequency of administration.
Our study has several limitations. It is cross-sectional with all the statistical limitations associated with this type of study design. We only selected non-chain owned HD units, primarily because there was an ease of access to obtain the data. Since participation in the study was voluntary, data from some countries were very limited, and also some of the participating countries have data from only one HD unit. The survey was not designed to evaluate the effectiveness of any of the drugs used for the treatment of anemia.
In conclusion, from the group of patients analyzed, despite the high prevalence of those receiving ESAs and IV irons, a significant fraction of them had Hb levels out of the range recommended by the RPCA-ERC/SLANH. Possible contributing factors and solutions to improve this problem require further investigation. Therefore, we are now beginning to re-collect data for the updated survey continuation that will allow us to increase the number of participating units and as a consequence, the number of the HD patients. Additionally, this updated and expanded survey will make a significant contribution in strengthening the commitment and understanding of the management of anemia in HD patients in LA.
Acknowledgements
ARGENTINA: Administradora de Salud SRL: Dr. Hugo Ramírez; CENCOS (Centro Nefrológico Coronel Suárez): Dr. Carlos Alberto Alberdi; CENDICA CADRA: Dr. Gordo Domingo Ponce, Dr. Arturo Guillermo Arias; Centro del Riñón SRL: Dr. Norberto Gómez; Centro Hemodiálisis GA: Dra. Norma Gladys Nieto; Centro Nefrológico (Centro de Diálisis) Balcarce: Dra. Stella Trevisán, Dr. Denis Allende; Centro Nefrológico Integral: Dr. Ricardo Felix Tosello; Centro Privado de Diálisis Tandil SA: Dr. Vito Mezzina; CEPER San Francisco SH: Dra. María Luisa Favaro; CEPER Unidad Renal Arroyito: Dra. Alicia Lonatti; CEPER Unidad Renal las Varillas: Dr. Jorge Rubiolo; Clínica Privada Vélez Sarsfield: Dr. Rafael Alberto Maldonado; Diálisis Madariaga: Dr. Estaban Lucas Siga; Hospital Aeronáutico Central: Dr. Carlos Blanco; Hospital Británico de Buenos Aires: Dra.Vanesa Sabrina Pomeranz, Dr. Mariano Forresteri, Dr. Fernando Juan Lombi, Dra. Romina Paola Iriarte, Dr. Vicente Martin Campolo Girard, Dr. Hernán Trimarchi; Hospital Churruca Visca: Dra.Liliana Andrade; Instituto de Nefrología Pergamino (Sucursal Rojas) (N1): Dra. Ana María Cusumano; Instituto de Nefrología y Hemodiálisis SRL: Dr. Miguel A. Discépolo; Instituto Pergamino SRL (N2): Dra. Ana María Cusumano; Nefrología HAEDO SRL: Dra. Alicia Carlevaro; Salud Renal SRL San Luis: Dra. Paula Gabriela Arenas; Se Ne Ml Srl: Dr. Carlos D. Braccalenti; Servicios de Nefrología Clínica Privada Mayo: Dr. Luis Eduardo Rivera; Servicio de Nefrología y Hemodiálisis ANJOR SRL: Dra. Norma Nieto; SOLANEF SRL (San Francisco Solano Buenos Aires): Dr. Carlos Cangado Pousa; Unidad 2115: Dr. Sergio Panese; Unidad Clínica Avenida: Dr. Daniel E. Arrudi; Unidad FAERAC: Dr. Eduardo Meneguzzi; Unidad Renal Hospital Italiano: Dra. Marcela Alejandra Fernández; Unidad Renal Rio Cuarto: Dra. Cecilia Grahovac. BOLIVIA: Hospital Obrero N 1 (La Paz): Dr. Roberto Barriga Arroyo. BRASIL: Associação Renal Vida Blumenau: Dr. Humberto Rebello Narciso; Centro de Nefrología do Maranhão CENEFRON: Dr. Natalino Salgado; Centro de Terapia Renal de Itaboraí (CTRI): Dra. Elisa Albuquerque; Centro de Tratamento Renal: Dra. Lucíla María Valente; Clínica de Medicina Interna e Nefrología: Dra. Patrícia Ferreira Abreu; Clínica de Nefrología de Sergipe CLINESE: Dr. Kleyton de Andrade Bastos; Clínica de Tratamento Nefrológico: Dra. Silvana Mourão; CLIRE Clínica de Doenças Renais Ltda: Dra. Rosely Riki Matsubara; Dialise do Hospital das Clínicas da Faculdade de Medicina de Botucatu: Dra.Jacqueline Teixeira Caramori; Fundação Oswaldo Ramos: Dra. Maria Eugênia Fernandes Canziani; GAMEN: Dra.Maria da Gloria Mesquita Santiago Lima; Hospital Universitario Presidente Dutra HUPD: Dr. Natalino Salgado; Instituto de Doenças Renais LTDA (Rio Grande do Sul): Dr. Fernando Saldanha Thomé, Dr. Elvino Barros; Instituto de Hemodiálise Sorocaba: Dr. José Luís Bevilacqua; Instituto do Rim do Paraná: Dr. Sérgio Gardano Elías Bucharles; Renaclin Clinica de Doenças Renais: Dra. Márcia Bastos; Unicom - Sociedade de Nefrología Ltda: Dr. Fernando Frattini Neto; Unidade de Tratamento Dialítico: Dr. Waldir Eduardo Garcia. CHILE: Centro de Diálisis Clínica Antofagasta: Dr. Eric Zúñiga Saravia; Centro de Diálisis Hospital Clínico Fusat: Dra. Anna María Arévalo Arévalo; Centro de Diálisis Tome Ltda Sucursal Penco: Dr. José Luis López, Dra. Claudia Cerna; Centro de Diálisis Vallenar Ltda: Dr. Enrique Quintana Meneses; Centro Hemodiálisis MEDICEN: Dra. María Eugenia Estévez Molina; Centro Médico y Diálisis TilTil Ltda: Dra. Ana Sota; Centro Nefrológico de Diálisis TOME Ltda: Dra. María José Aliaga, Dr. Jorge Morente Esquivel; Centro Regional de Diálisis Araucarias Thno Sucursal Hualpen: Dr. Jaime Lastra Salcedo; Centro Regional de Diálisis Ltda (Concepción): Dr. Jaime Lastra Salcedo; Centro Salud Anteres SA: Dr. Hernán E. Echeverría Torres; Clínica Alemana de Santiago SA: Dr. Enrique Reynolds H.; Clínica Dávila: Dr. Claudio González Torres; Clínica Las Condes: Dr. Rodrigo Orozco; Corporación Paul Harris: Dr. Antonio Saffie Ibáñez; Diálisis Ñuñoa: Dr. Luis Eduardo Ortiz Brieva; Diálisis Calama Ltda: Dr. Fernando González; Diálisis Clínica Indisa: Dr. Andrés Boltansky; Diálisis Hospital Regional Rancagua: Dr. Juan Romero Tomasevich; Diálisis Los Andes Ltda: Dr. Juan Lemus; Diálisis Recoleta: Dra. María Eugenia Sanhueza; Hospital Carlos Van Buren: Dr. Hugo Poblete Badal; Hospital Dr Hernán Henríquez Aravena: Dr. Arturo Pinto Zabaleta; Hospital Exequiel González Cortés: Dr. Jean Grandy Henríquez; Hospital Luis Calvo Mackenna: Dra. Ana María Lillo; Hospital Puerto Natales: Dra. Paulina Folch; Hospital de Coquimbo: Dra. Doris Subiabre Cortes; Hospital Naval Almirante Nef: Dr. Gonzalo Silva Rojas; Rancagua Dial Ltda: Dr. Héctor Labbe Saffa; Saludial Concepción y Saludial Coronel: Dr. Jorge Münzenmayer B; Sermidial Valparaiso: Dra. Esperanza Merchán Molina; Sermidial Viña del Mar: Dra. María Carlina Vargas Serrano; Unidad de Diálisis Hospital Ancud: Dra. Katia Velásquez Martínez; Unidad de Hemodiálisis Hospital Regional Copiapó: Dr. Guillermo Callejas González; Vitta Dial Los Andes: Dr. Pablo Merchán Córdova. COLOMBIA:Biorrenal: Dr. Jaime José Torres Saltarín. ECUADOR: Clínica de los Riñones MENYDIAL: Dr. Darío Jiménez Acosta; Instituto Ecuatoriano de Diálisis y Trasplante IEDYT: Dr. Fabián Ortiz: Herbener. HONDURAS: Unidad de Hemodiálisis IHSS (Tegucigalpa): Dr. Gaspar Rodríguez M. MÉXICO: Centro Hospitalario la Concepción de Saltillo: Dr. Mario Gastón Melo, Dra. María Dolores del Bosque Saucedo; Hemodiálisis Hospital San José Tec de Monterrey: Dr. Alejandro Valdés Cepeda; Hospital Central Dr. Ignacio Morones Prieto: Dr. Francisco Gerardo Alfaro Abundiz, Dr. Jesús Martínez Reyna; Hospital General Zona # 2: Dr. Alfonso Ramos Sánchez; Hospital Regional de Zona IMSS: Dra. Patricia Muñoz Sotelo; Hospital Star Medica Morelia: Dr. Eduardo Quintana Piña; Hospital Universitario José Eleuterio González: Dr. Edgar Marcelo Arellano Torres; Instituto Mexicano del Seguro Social (IMSS) HGZ N 8: Dr. Javier Piñón Escobedo; Instituto de Seguridad y Servicios Sociales de los Trabajadores ISSSTE: Dr. Javier Piñón Escobedo; Subsección de Hemodiálisis Hospital Central Militar: Dr. Ricardo Mendiola Fernández, Dr. Gustavo A. Martínez; Unidad de Hemodiálisis Hospital General Regional No. 25 del IMSS: Dr. Antonio Méndez Durán; Unidad Renal Ambulatoria: Dr. Javier Piñón Escobedo. NICARAGUA:Hospital Salud Integral: Dra. Nubia Cano. PANAMÁ:Unidad Hospital Susana Jones Cano: Dra. María Niedda. PARAGUAY: Departamento de Nefrología Hospital de Clínicas FMC-UNA: Dra. Elvira Giménez Rolón; Hospital Regional Encarnación: Dr. Darío Cuevas; Hospital Nacional: Dra. María Concepción Ríos, Dra. Miriam López; Servicio de Nefrología Hospital Central de IPSS: Dr. Roger Aureliano Ayala Ferrari.PE RÚ:Centro Nefrológico SA Cenesa: Dr. Leónidas Carrillo; Hospital Centro Médico Naval: Dr. Ernesto Valencia Valz; San Fernando SAC: Dr. Pedro Castillo Rodríguez. PUERTO RICO: Hospital Renal Universitario: Dr. Pedro Carlos Pagan Torres. REPÚBLICA DOMINICANA: Hospital Regional Dr. Antonio Musa Unidad de Diálisis: Dra. Sandra Rodríguez Manzano. URUGUAY: Uruguayana: Dra. Nieves Campistrús; Asociación Médica de San José: Dr. Alejandro Ferreiro; Centro de Diálisis Crónica Hospital Maciel: Dra.Laura Solá; Centro Nefrológico Integral Camedur: Dra. Virginia Matonte; COMERO (Rocha): Dra. Cecilia Tognola; COMEPA: Dra. Mariela Odriozola, Dra. Marta Pereyra, Dra.Marcela Daglio; Diálisis del Hospital Británico: Dr. Juan Fernández Cean, Dra. Catalina Gutiérrez; INU: Dra. Enriqueta Carbonell; SARI- IBIRAPITA: Dra. Alicia Petraglia, Dra. Analía Varela; SE.NE.C.C. (Centro de Diálisis de COMECA): Dra. Lydia Zampedri; Unidad CEDINA: Dr. Juan Carlos Díaz, Dra. Aquelina Álvarez. VENEZUELA: Unidad Hospital Núñez Tovar: Dr. Carlos Márquez, Dr. Andrés Cortez; Hospital Universitario de Los Andes Mérida: Dr. Bernardo Fargier, Dra. Dulce Winterdaal; Diálisis San Antonio CA: Dr. Silvano Vernamonte Damiani; Unidad de Diálisis GAPB Guatire: Dr. Leoncio Serrano; Unidad de Diálisis Hospital Militar Caracas: Dr. Leoncio Serrano Centro Médico Docente La Trinidad: Dra. Ana María Sanánez; Centro Nefrológico Integral Falcón: Dr. Ramón Polanco; Hospital de Clínicas Caracas: Dra. Julia Bravo, Dra. Leonor Oliveros, Dr. Pablo Amair; Servicios Nefrológicos CA: Dra. Belquis Chuecos; Unidad de Hemodiálisis Naguanagua: Dr. David López, Dr. Orleans Méndez; Unidad de Hemodiálisis Jesús de Nazareno CA N 1: Dra. Silvia Villarroel; Unidad de Hemodiálisis Jesús de Nazareno CA N 2: Dra. Silvia Villarroel; Unidad Diálisis Mérida (DIAMERCA): Dr. Bernardo Fargier, Dr. Luis Guilarte.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
References
1. Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. for the CHOIR Investigators. Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease. N Engl J Med 2006;355:2085-98. [ Links ]
2. Drüeke TB, Locatelli F, Clyne N, Eckardt K-U, Macdougall IC, Tsakiris D, et al. for the CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006;355:2071-84. [ Links ]
3. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU et al.; TREAT Investigators. A trial of darbepoetinalfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009;361(21):2019-32. [ Links ]
4. Coyne DW. The health-related quality of life was not improved by targeting higher hemoglobin in the Normal Hematocrit Trial. Kidney Int 2012;82:235-41. [ Links ]
5. KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease, 2007 update of hemoglobin target. Am J Kidney Dis 2007;50(3):471-530. [ Links ]
6. Locatelli F, Covic A, Eckardt K-U, Wiecek A, Vanholder R, on behalf of the ERA-EDTA ERBP Advisory Board. Anaemia management in patients with chronic kidney disease: a position statement by the Anaemia Working Group of European Renal Best Practice (ERBP). Nephrol Dial Transplant 2009;24:348-54. [ Links ]
7. Carlini R, Campistrús N, Andrade L, Bregman R, Chifflet L, Correa-Rotter R, et al. Recommendations for the clinical practice of the Latino American Society of Nephrology and Hypertension (SLANH) for the treatment of anemia in patients with chronic renal disease (RPCA-ERC/SLANH). Nefrología Latinoamericana 2009;13:1-30. [ Links ]
8. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl 2012;(2):S299-310. [ Links ]
9. Cusumano AM, Gonzalez Bedat MC, García-García G, Maury Fernandez S, Lugon JR, Poblete BH, et al. Latin American Dialysis and Renal Transplant Registry: 2008 Report (data 2006). Clin Nephrol 2010;74 Suppl 1:S3-8. [ Links ]
10. Sesso R, Lopes AA, Thomé FS, Bevilacqua JL, Romão JE Junior, Lugon J. Brazilian Dialysis Census Report 2008. J Bras Nefrol 2008;30(4):233-8. [ Links ]
11. Gurgel TC, Cherchiglia ML, Acurcio F de A, Szuster DA, Campos DA, Gomes IC, et al. Erythropoietin use by incident HD patients in the Brazilian Unified National Health System, 2002-2003. Cad Saude Publica 2012;28(5):856-68. [ Links ]
12. González C, Ferreiro A, Schwedt E, Pinato M. 2009 Uruguayan Register Dialysis. Available at: http://www.nefrouruguay.com/. [ Links ]
13. Poblete H. Chilenan Nephrology Society. Dialysis Register 2010. Available at: http://www.nefro.cl/registros/registro-de-hemodialisis.html. [ Links ]
14. Tsubakihara Y, Nishi S, Akiba T, Hirakata H, Iseki K, Kubota M, et al. 2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ther Apher Dial 2010;14(3):240-75. [ Links ]
15. National Institute for Health and Clinical Excellence (NICE). Anaemia management in people with chronic kidney disease. (CG114). 2011. Available at: http://guidance.nice.org.uk/CG114. [ Links ]
16. Gilg J, Webb L, Feest T, Fogarty D. UK Renal Registry 13th Annual Report (December 2010): Chapter 9, haemoglobin, ferritin and erythropoietin amongst UK adult dialysis patients in 2009: national and center-specific analyses. Nephron Clin Pract 2011;119 Suppl 2:c149-77. [ Links ]
17. US Renal Data System, USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2012. Available at: http://www.usrds.org/atlas.aspxcion. [ Links ]
18. Hasegawa T, Bragg-Gresham JL, Pisoni RL, Robinson BM, Fukuhara S, Akiba T, et al. Changes in anemia management and hemoglobin levels following revision of a bundling policy to incorporate recombinant human erythropoietin. Kidney Int 2011;79(3):340-6. [ Links ]
19. Bamgbola OF. Pattern of resistance to erythropoietin stimulating agents in chronic kidney disease. Kidney Int 2011;80(5):464-74. [ Links ]
20. Elliot J, Mishler D, Agarwal R. Hyporesponsiveness to erythropoietin: causes and management. Adv Chronic Kidney Dis 2009;16(2):94-100. [ Links ]
![]() Correspondence:
Correspondence:
Raúl Carlini
Division of Nephrology
University Hospital of Caracas
Caracas, Venezuela
raul.carlini@gmail.com
Enviado a Revisar: 17 Sep. 2012
Aceptado el: 14 Oct. 2013