Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.21 no.6 Madrid nov./dic. 2006
ORIGINAL
Effects of non steroidal anti-inflammatory drugs on the antioxidant defense system and the membrane functions in the rat intestine
Efectos de los fármacos aniinflamatorios no esteroideos sobre el sistema de defensa antioxidante y las funciones de membrana en el intestino de rata
P. Nair, S. Singh Kanwar and S. Nath Sanyal*
Department of Biophysics. Panjab University. Chandigarth. India.
ABSTRACT
In the present study the effects of two cycloxygenase-2 (COX-2) selective inhibitors, celecoxib and nimesulide as compared to a non-selective COX inhibitor, aspirin was studied in the rat intestine. Female Wistar rats weighing between 150-175 g were divided into four groups having 8 animals each as follows: Group 1(Control), Group 2- Aspirin (40 mg/kg), Group 3- Nimesulide (10 mg/kg) and Group 4- Celecoxib (10 mg/kg). After 35 days of treatment the animals were sacrificed, intestine removed and the effects on the antioxidant defense system, membrane composition and functions along with the membrane specific enzymes were studied in different regions of the intestine. The study showed a significant increase in the lipid peroxide levels as TBA-reactive substance as well as the conjugated dienes, except for celecoxib treated group which showed a decrease. Significant decrease was also observed in the level of reduced glutathione (GSH), superoxide dismutase (SOD), glutathione-s-transferase and catalase activities for aspirin and nimesulide group while Celecoxib caused an increase in glutathione reductase (GR). Aspirin and nimesulide exhibited an increase in the brush border membrane (BBM) bound enzyme activities like sucrase, lactase, maltase and alkaline phosphatase in the small intestine while celecoxib showed decrease in lactase, maltase and alkaline phosphatase. The phospholipid content increased only for aspirin treated group while cholesterol decreased in all the treatment groups. Also celecoxib treatment brought about an increase in glycolipid content. The membrane fluidity was studied by the rotational diffusion of 1, 6, diphenyl, 1, 3, 5 hexatriene (DPH) incorporated in the membrane and the fluorescence polarization (p), fluorescence anisotropy(r), anisotropy parameter [r0/r -1]-1 and order parameter [S2 = (4/3r – 0.1)/ r0] were recorded. No significant change in the fluorescence parameters were observed in the BBM and the liposomes made from the BBM lipids for the treatment groups. These results indicate that celecoxib may be accepted as a safer drug in terms of overall gastro-intestinal toxicity as compared to the aspirin and nimesulide.
Key words: Non-steroidal anti-inflammatory drugs. Oxidative stress. Intestinal membrane. Rat.
RESUMEN
En el presente estudio se comparaban los efectos de dos inhibidores selectivos de la ciclo-oxigenasa-2 (COX-2), celecoxib y nimesulide, con los de un inhibidor no selectivo de la COX, la aspirina, en el intestino de la rata. Se escogieron ratas Wistar hembra, con un peso de 150-175 g, y se las dividió en 4 grupos, con 8 animales cada uno, como sigue: Grupo 1 (Control), Grupo 2 – Aspirina (40 mg/kg), Grupo 3 – Nimesulide (10 mg/kg), y Grupo 4 – Celecoxib (10 mg/kg). Tras 35 días de tratamiento, se sacrificó a los animales, se extirpó el intestino, y se estudiaron los efectos sobre el sistema de defensa antioxidante, la composición de la membrana y sus funciones, además de las enzimas de membrana específicas, en diferentes regiones del intestino. El estudio mostró un aumento significativo de las concentraciones de peróxido lipídico como sustancia TBA reactiva así como de dienos conjugados, excepto para el grupo tratado con celecoxib que mostró un descenso. También se observó un descenso significativo en las actividades de glutatión reducido (GSH), de superóxido dismutasa (SOD), de glutatión-s-transferasa y de catalasa en los grupos tratados con aspirina y con nimesulide, mientras que el grupo tratado con celecoxib tuvo un aumento de la glutatión reductasa (GR). La aspirina y el nimesulide mostraron un aumento de las actividades de las enzimas ligadas a la membrana de borde en cepillo (BBM) como la sucrasa, lactasa, maltasa y fosfatasa alcalina. El contenido en fosfolípidos aumentó sólo en el grupo tratado con aspirina mientras que el colesterol disminuyó en todos los grupos de tratamiento. En el grupo de celecoxib también se objetivó un aumento en el contenido en glucolípidos. Se estudió la fluidez de la membrana mediante difusión rotacional del 1,6-difenil-1,3,5-hexatrieno (DPH) incorporado a la membrana y se registraron la polarización de fluorescencia (p), la anisotropía de fluorescencia (r), el parámetro de anisotropía [r0/r -1]-1 y el parámetro de orden [S2 =(4/3 r – 0,1)/ r0]. No se observaron cambios significativos en los parámetros de fluorescencia en la BBM ni de los liposomas formados a partir de los lípidos de la BBM en ninguno de los grupos tratados. Estos resultados indican que se puede considerar al celecoxib como un fármaco más seguro en cuanto a toxicidad gastrointestinal general en comparación con el nimesulide y la aspirina.
Palabras clave: Fármacos antiinflamatorios no esteroideos. Estrés oxidativo. Membrana intestinal. Rata.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used to relieve pain and symptoms of arthritis and soft tissue inflammation. The use of NSAIDs is limited by their tendency to cause mucosal damage in the gastrointestinal tract. Aspirin, nimesulide and celecoxib belong to three different groups of NSAIDs with difference in cycloxygenase (COX) inhibition wherein nimesulide and celecoxib act as COX-2 selective inhibitor while aspirin as a non selective inhibitor for COX enzyme. NSAIDs act to block the first phase of prostaglandin synthesis by binding to and inhibition of COX conversion of arachidonic acid (AA) to PGG2 1. Aspirin is an exception where it inhibits due to irreversible acetylation of cycloxygenase component of COX, leaving peroxidase activity unaffected2.
Aspirin relieves the pain by inhibiting the synthesis of prostaglandins that prevents the sensitization of pain receptors by different stimulants, which appear to mediate pain response3-5. Clinical and epidemiological evidence suggest that aspirin produces dose related gastrointestinal toxicity6-8 that is sometimes fatal8. Although nimesulide also inhibits inflammation, various nonprostaglandin mechanisms have been proposed to explain its mode of action: inhibition of 1) histamine release and activity9, 2) cytokine release10. In clinical trials it has shown adverse reaction in gastrointestinal system but is reversible upon cessation of the drug11-13. Celecoxib as compared to these two drugs is highly COX-2 selective and has the same mode of action. Patients with significant risk factors for cardiovascular events (e.g. hypertension, hyperlipidaemia, diabetes mellitus, and smoking) should only be treated with celecoxib after careful consideration. Studies have shown that celecoxib inhibits the progression of colon tumor in human and animal models and inhibits the in vitro growth of several other tumor cell types14-16. Gastrointestinal (GI) problems are far less common with celecoxib than with other NSAIDs. This is due to the fact that celecoxib is highly COX-2 selective and therefore less likely to cause gastric damage. Celecoxib can lead to liver damage. Other rare but serious side effects of celecoxib include allergic reactions and kidney problems.
All the three drugs have been shown to cause intestinal toxicity at different levels. Toxicity may be due to initial biochemical modifications in the brush border membrane or due to alteration in the intestinal mucosal architecture. In the present experimental work COX-2 selective inhibitors (nimesulide and celecoxib) and nonselective COX inhibitors (aspirin) were studied for their influence on the oxidative stress status, membrane functions, lipid profile and lipid fluidity in rat intestine.
Materials and methods
Animals and treatment
Female Wistar rats weighing between 150-175 g were obtained from the Central Animal House, Panjab University, for the experimental work, strictly in accordance with the guidelines as outlined by the institutional ethics committee. After acclimatizing for 1 week, animals were divided into four groups having 8 animals each as follows: Group 1(Control), Group 2- Aspirin (40 mg/kg), Group 3- Nimesulide (10 mg/kg) and Group 4- Celecoxib (10 mg/kg). After 35 days of treatment the animals were sacrificed under an overdose of ether anesthesia. In order to avoid diurnal variation, the animals were uniformly sacrificed around 8 a.m. throughout the study. From each animal intestine was removed and divided into duodenum, jejunum, ileum and colon. Each segment was flushed with ice cold saline, weighed and proceeded for the reported parameters.
A 10% homogenate of the intestinal segments was prepared in chilled 1mM Tris-50 mM Mannitol buffer (pH 7.4). The homogenate was centrifuged at 1,000 g for 10 min at 4o C. Pellet was discarded and the supernatant used for various biochemical estimations. A portion of 1,000g supernatant was again centrifuged at 10,000 g for 20 min to obtain post mitochondrial supernatant (PMS) which was further used for biochemical estimations. The brush border membrane (BBM) of different segments of rat intestine was isolated using the method of Schmitz et al (1973)17. The 10% homogenate was passed through two layers of cheese cloth. Anhydrous CaCl2 was added with constant stirring (10 mM final conc.) to the above filtrate & left for 10-15 min in cold. It was centrifuged at 2,000 g for 10 min at 4o C. The supernatant obtained was centrifuged at 42,000 g for 20 min. The pellet was suspended in 20 vol. of 50 mM sodium maleate buffer (pH 6.5-6.8). The supernatant was discarded. The suspended pellet was centrifuged at 42,000 g for 20 min. The pellet obtained was suspended in 50 mM sodium maleate buffer (pH 6.5-6.8) containing 0.02% sodium azide (NaN3). The final membranes so obtained were used for prepa-Hypophosphatemia: control by nutritional teams ring liposomes from the extracted lipids and for various biochemical as well as fluorescence studies. The BBM prepared above was free from mitochondria, microsomes, lysosomes, basolateral membranes and nuclei as assessed by the marker enzymes.
Disaccharidase assays: Sucrase, Lactase and Maltase
The activities of these 3 enzymes were determined by measuring the D-glucose liberated from the respective sugar substrate using Glucose oxidase-Peroxidase enzymatic system (GOD-POD)18.
Alkaline phosphatase assay
Alkaline phosphatase activity was assayed according to the method of Bergmeyer19 where p-nitrophenyl phosphate was used as the substrate. Alkaline phosphatese acts on this by hydrolyzing it to yield p-nitrophenol. The yellow colour was measured at 410 nm.
Lipid peroxide (LPO)
Lipid peroxide formation was assayed by the method of Wills20. Since malonyldialdehyde is a degradation product of peroxidised lipids, the development of pink colour with the absorption characteristics (Absorption maximum at 532 nm) as a TBA-MDA chromophore has been taken as an index of lipid peroxidation.
Glutathione estimation
Glutathione content was estimated according to the method of Ellman21. In this method 5, 5-Dithiobis 2- Nitrobenzioc acid (DTNB) is reduced by –SH groups to form 1 mole of 2-nitro-5-mercapto benzoic acid per mole of SH.
Glutathione S- transferase (GST)
The enzyme was assayed by the method of Habig et al22. GST catalyses the formation of the glutathione conjugates of CDNB which absorb maximum at 340 nm and have an extinction coefficient of 9.6 m M-1 cm-1.
Glutathione reductase (GR)
The enzyme was assayed by the method of Massey and Williams23. The utilization of NADPH is directly related to the activity of GR.
Catalase
Catalase was estimated in an U. V. spectrophotometer by the method described by Luck24. H2O2 was used as substrate. The absorption of H2O2 solution is measured at 240 nm on decomposition of H2O2 with catalase absorption that decreases with time which is recorded and from this decrease in optical density, the enzyme activity calculated.
Superoxide dismutase (SOD)
Superoxide dismutase assay was performed according to the method of Kono et al25. The reduction of Nitro Blue Tetrazolium (NBT) to a blue color formation mediated by hydroxylamine hydrochloride was measured under aerobic conditions.
Extraction of lipids
Lipids were extracted from the BBM following the method of Folch et al26. Membrane suspension (150-200 mg protein) was mixed in a flask with 20 vol of chloroform: methanol (2:1 v/v) and left for 15 min at 45° C. The contents were mixed thoroughly and filtered through a Whatman No1 filter paper into a graduated cylinder. The residue left on the filter paper was then washed three times with 10 ml of chloroform: methanol (2:1). Then, 0.2 vol KCl (0.9%) was added (20% of total volume) to the extract. The contents were mixed vigorously and allowed to stand in cold overnight so as to separate the aqueous and lipid layers distinctly well. Upper aqueous phase was removed with Pasteur pipette and the lower layer washed three times with 2 ml chloroform: methanol: 0.9% KCl 3: 48:47 v/v. The washed lower layer was transferred to a round bottom flask and evaporated to dryness at a temp below 45° C while the upper aqueous layer was added each time to the previously separated upper phase. To the residue, 5 ml of chloroform: methanol: water, 64:32:4 v/v was added and evaporated to dryness. This was repeated three times. The dried lipid was redissolved in chloroform and filtered again. The filtrate was evaporated in a rotary evaporator under reduced pressure and at a temp slightly less than 45° C. A known volume of chloroform: methanol (2:1 v/v) was added to redissolve the lipids in a tightly closed container and used as such for various lipid estimations.
Estimation of total Lipids
Total lipids were estimated following the method of Fringes and Dunn27 measuring the coloured complex with a phosphate ester of vanillin (colouring reagent).
Estimation of cholesterol
In the presence of H2SO4 and Glacial acetic acid, cholesterol forms a colored complex with FeCl3 that can be measured colorimetrically at 540 nm28.
Estimation of Glycolipids
Estimation of hexose unit found in conjugation with lipids in the glycolipids was done by the method of Dubious et al29.
Estimation of phospholipid phosphorus
Inorganic phosphorous estimation was done in the phospholipids after digestion according to the method of Ames (1966)30.
Estimation of ganglioside-sialic acid
Sialic acid was estimated by the method of Warren31. Sialic acid (N acetyl neuraminic acid) is oxidized with Sodium periodate in conc Orthophosphoric acid. The periodate oxidation product is coupled with Thiobarbituric acid and resulting chromophore is extracted in Cyclohexanone.
Conjugated diene estimation
Conjugated diene content in the sample was estimated following the method as described by Lakshmi and Balasubramanian32. 50 μl of redissolved lipids in 2:1 Chloroform: Methanol mixture was taken in different test tubes and dried completely at 37° C. To this 1 ml of sodium maleate buffer was added and conjugated diene content was estimated in them by measuring the absorbance at 233 nm, and the amount calculated using the molar extinction coefficient of 2.52 × 104M-1cm-1.
Liposomes preparation
Liposomes were prepared from the extracted lipids by the method of Schachter and Shinitzky33. Extracted lipid was suspended in sodium maleate buffer to a final concentration of approximately 0.3 mg/ml and the mixture was sonicated for 10 min using a bath type sonicator. Thereafter, the suspensions were centrifuged for 10 min at 50,000 g in a Beckmann Coulter ultra centrifuge. The supernatant liposomes were tested for membrane fluidity with the fluorescent probe (1, 6-diphenyl-1, 3, 5- hexatriene) as described for the membranes in the following.
Fluorescence studies
The lipid-soluble fluorescent probe, 1, 6-Diphenyl- 1, 3, 5-hexatriene (DPH) was used in the fluidity studies. For this a stock solution of 2 mM probe in tetrahydrofuran (THF) was prepared and stored being protected from light at room temp. Aqueous suspension of DPH was prepared freshly each time. A small volume of DPH solution in THF was injected with rapid stirring into 1,000 volumes of sodium maleate buffer at room temperature. The suspension was stirred for at least 2 hr after which no odor of THF was detected and the suspension showed negligible fluorescence. In a typical experiment BBM (100-200 μg protein) and liposomes were incubated in 2 ml of sodium maleate buffer containing 1 μM DPH suspension for 2-4 hr at 37° C. Thereafter, estimations of fluorescence intensity (F), fluorescence polarization (p) and fluorescence anisotropy (r) were made with an excitation wavelength of 365 nm and emission wavelength of 430 nm using a Perkin Elmer Luminescence Spectrometer LS 55. Anisotropy parameter [ro/r -1]-1 was then calculated using ro value for DPH as 0.36234. Also the order parameter was calculated using the relationship S2 = (4/3 r – 0.1)/ ro35.
Statistical analysis
Statistical analysis of the data was performed by analysis of variances (one way ANOVA) following one way ANOVA post-Hoc test using least significance difference (LSD).
Results
A highly significant increase in the lipid peroxidation was observed in the duodenum region of the aspirin treated group whereas celecoxib showed a fairly significant decrease (table I). A fairly significant increase was reported in the jejunum and ileum segment of the aspirin treated group. No alteration in lipid peroxides was found in the colon region following any of the treatments.
In the duodenum region of intestine of aspirin treated group a significant decrease in the reduced glutathione level was observed whereas a significant increase recorded in the celecoxib (table II). In the jejunum region aspirin treated group showed a significant decrease (p < 0.01) while celecoxib caused a significant increase. Aspirin treated group showed a fairly significant decrease whereas nimesulide showed no significant change and celecoxib a non significant increase in the glutathione level in the ileum region. Colon region showed a significant decrease in the glutathione level for the aspirin and nimesulide treated groups.
Aspirin treatment brought about a significant decrease in GST activity in the duodenum region while nimesulide administration caused a significant increase (table III). Jejunum showed a highly significant increase for the celecoxib treated group. A highly significant decrease was observed in the ileum region of both aspirin and nimesulide treated groups, respectively. The colonic segment also showed a highly significant decrease in the enzyme activity for the aspirin and nimesulide treated groups.
Aspirin and celecoxib treated groups witnessed a fairly significant decrease in the SOD level in the duodenum segment (table IV). Jejunum region showed a fairly significant decrease in the aspirin treated group. SOD level in the other two treated groups was found to be normal as compared to the control in the jejunum region. The ileum region followed the same trend as in the jejunum in all the treated groups. The colon region observed a highly significant decrease for the aspirin treated group. A fairly significant decrease was also reported in the same region for the nimesulide treated group.
Table V shows the effects of aspirin, nimesulide and celecoxib on the intestinal catalase activity. Duodenum region showed a fairly significant decrease in catalase in case of the aspirin treated group. A fairly significant decrease in the enzyme activity was found in the ileum region of aspirin as well as that of the nimesulide treated groups. The colon region was reported to have a fairly significant decrease for aspirin and nimesulide.
A fairly significant decrease in the Glutathione reductase activity was found in the duodenum region of intestine in the case of aspirin treated group while a fairly significant increase was found in the celecoxib treated group (table VI). Aspirin treatment brought about a fairly significant decrease in the jejunum region of intestine. For ileum, enzyme activity was reduced fairly significantly for the aspirin treated group. Aspirin administration brought about a fairly significant increase in the sucrase activity in the duodenum and jejunum and a highly significant increase in the ileum region (table VII). The sucrase activity remained unaffected in all the regions of small intestine of nimesulide and celecoxib treated groups.
Duodenum region witnessed a highly significant increase in the lactase activity in the aspirin treated group whereas a highly significant decrease was observed in celecoxib (table VIII). A significant rise in the lactase activity was seen in the jejunum region for the aspirin treated group. In the ileum a fairly significant increase was recorded in all the treated groups. Celecoxib group brought about a highly significant decrease in the maltase activity in the duodenum region (table IX). In the jejunum fairly significant increase was found for the aspirin treated group while the nimesulide and celecoxib showed a significant decrease in the same region. Nimesulide and celecoxib treatment brought about a highly significant decrease in the maltase activity in the ileum region.
A highly significant increase in the alkaline phosphatase activity in the duodenum region was found for the aspirin treated group (table X). Nimesulide showed a fairly significant increase while celecoxib showed a fairly significant decrease in the same intestinal region. Jejunum witnessed a highly significant increase for the aspirin treated group whereas a fairly significant decrease was found for the celecoxib. In the ileum a highly significant increase in the enzyme activity was recorded in favor of the aspirin and nimesulide treated groups, respectively, while celecoxib showed a highly significant decrease. Alkaline phosphatase activity remained unaffected in the colon region of intestine of all the treated groups.
Table XI shows the results of the effects of aspirin, nimesulide and celecoxib administration on the total lipids in the intestinal BBM. A highly significant decrease in total lipids was observed in the case of aspirin treated group. A fairly significant increase in the phospholipid composition in the intestinal BBM was also recorded. However, a significant decrease in cholesterol level was seen in the aspirin treated group. A highly significant decrease was observed in the case of nimesulide and celecoxib. The glycolipid composition showed a highly significant increase in the intestinal BBM of the celecoxib treated group. No significant change was observed in the case of other treated groups. Also, for the ganglioside composition no significant change was observed.
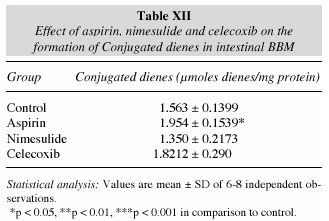
The occurrence of conjugated dienes is a mark of lipid peroxidation in the membranes. A fairly significant increase in the diene formation was noticed in the intestinal BBM of the aspirin treated group while no alterations observed in nimesulide and celecoxib (table XII). Aspirin treatment produced the highest absorbance corresponding to the conjugated dienes followed by nimesulide while celecoxib produced the decrease in absorbance when compared to the control (fig. 1).
The treatments of NSAIDs produced no significant alterations in the fluorescence studies related to the parameters like fluorescence polarization, anisotropy and subsequently no change in the calculated anisotropy parameter and the order parameter values observed in the intestinal BBM (table XIII) and liposomes (table XIV). The fluorescence intensity was found to be maximum in aspirin treated BBM and greater than control. Whereas, nimesulide treated BBM resulted in lesser intensity peak than control while celecoxib BBM showing the peak corresponding to the least intensity among all the treatments (fig. 2).
Discussion
Results from the present studies have shown that an increase in lipid peroxidation occurs in different intestinal segments in NSAIDs treatment except in celecoxib. Earlier studies have also reported an increase in MDA levels during NSAIDs treatment36. Celecoxib showed a decrease in the lipid peroxidation in the duodenum Hypophosphatemia: control by nutritional teams region suggesting that the lower dose regimen to be safer against oxidative stress. The decrease in lipid peroxidation following celecoxib administration can be attributed to its high COX-2 selectivity as the later is known to induce lipid peroxidation in cellular systems. The level of glutathione is considered a critical determinant for the threshold of tissue injury. The importance of GSH is emphasized further by a study that showed a substantially disruptive effect on the mucosal architecture of pharmacological inhibition of GSH synthesis37. In the present study, no decline in GSH was observed in all the intestinal regions following nimesulide and celecoxib treatment while an enhanced level observed following aspirin treatment. This decrease in GSH levels attributes to the fact that GSH is being consumed in response of an increased lipid peroxidation during these drugs administration. Previous works have also reported a decrease in the GSH levels37,38 in intestinal tract following certain COX-2 non selective NSAIDs administration at higher doses. These results suggest that at a higher dose of COX-2 non selective NSAIDs, there is a greater risk of oxidative stress and hence intestinal injury. GST has GSH as its substrate and the decline in GST activity is in response to the dearth of substrate as GSH was also found to be low in small intestine as well as colon regions following aspirin and nimesulide treatment. However, celecoxib resulted in an increase in the GST activity.
Most of the NSAIDs are known to scavenge free radicals while certain NSAIDs like indomethacin have been reported to induce free radicals generation39. High level of COX-2 induced prostaglandins is known to be associated with the oxidative stress as COX-2 over expression induces the generation of free radicals and lipid peroxide formations. SOD and catalase are both antioxidant enzymes that function as blockers of free radical process40. In various previous studies SOD activity has been shown to decrease significantly in the GI mucosa after the administration of indomethacin38,41, diclofenac42 and ibuprofen43. Results of the present work have shown aspirin to decrease the level of SOD in all the small intestine regions as well as the colon. Further, the increase in the level of SOD in celecoxib treatment can be due to the highly COX-2 inhibitory nature and free radical scavenging capabilities of celecoxib that protects the cellular environment from reactive oxygen species by increasing the enzyme synthesis. Catalase activity was found to be decreased in the small intestine for aspirin and nimesulide treated groups.
Various reports have shown a decreased GR activity in the GI mucosa involving NSAIDs administration44. GR activity was found to be decreased in all the small intestinal regions in the aspirin treated group. Further, celecoxib caused an increased GR activity in duodenum region while causing no alterations in other small intestinal regions and colon. The increase in GR activity at low dose regimen of celecoxib can be seen as its protective mechanism whereby it increases the GR synthesis that helps in reducing the oxidized substrate (GSSG) by converting it into the reduced form (GSH) and thereby acting as an antioxidant.
The increased enzyme activities of the disaccharidases in small intestinal homogenates for aspirin and nimesulide group might be due to an increase in the number of molecular enzyme proteins. A rise in disaccharidases in the protein malnourished rats has also been described earlier45,46. The increased activity of disaccharidases was attributed to the high amount of carbohydrates present in a protein deficient diet. The substrate itself has a role to play in the stimulation of disaccharidase activities47-51. Mahmood et al52 reported that there is a two to three fold elevation in the sucrase and maltase activity in the protein calorie malnourished intestine (PCM) in undernourished monkeys as compared to the control animals. There was a decrease in lactase and maltase activity for the celecoxib dose in the present investigation. The alkaline phosphatase activity was however, increased for aspirin and nimesulide treated groups in the small intestine regions. A previous study by Singh et al53, reported an increase in the serum alkaline phosphatase activity after aspirin treatment and linked such increase with the hepatotoxic effects caused by aspirin. Thus, the increase in the alkaline phosphatase enzyme can be attributed to the self-protective mechanism of the system against the cytotoxicity of aspirin and nimesulide. Futhermore, the decline in alkaline phosphatase activity observed in small intestine following celecoxib treatment strongly favours its cytoprotective role.
Alterations in the lipid or protein composition may change the membrane fluidity, which is determined by lipid-protein interactions. Membrane fluidity is directly linked with membrane functions54. Aspirin and celecoxib treated groups showed no changes in total lipid or lipid: protein ratio as such. The total phospholipid content was found to be significantly increased in the nimesulide treated group. This increase in the phospholipid content under the effect of NSAIDs is supported by the fact that NSAIDs also increase the membrane fluidity55. Also changes in phospholipid content in the intestinal BBM can affect the membrane bound enzymes and permeability of the membrane to ions56. Further, increased phospholipid content makes the membrane more susceptible to peroxidation induced damages. Celecoxib did not alter the phospholipid content. Cholesterol content also decreased in all of the treatment groups. In a previous study57 it was reported that decrease in cholesterol phospholipid ratio in the intestinal BBM indicates an increase in fluidity. Celecoxib treatment has brought about an increase in glycolipids also. However, ganglioside composition noticed no significant changes for the treatment groups. Conjugated dienes are a measure of lipid peroxidation. The increase in amount of these in the present study in aspirin treatment indicates the extent of lipid peroxidation.
Previously fluorescence polarization methods have been applied increasingly to the study of biological membranes58-60. The particular usefulness of these methods stems from the fact that polarization of the fluorescence of a molecule depends upon the rate of molecular rotation of the lipid61. Binding of a fluorophore to biological macromolecule or membrane can be monitored by an increase in the polarization of fluorescence61. Similarly, since the rotation rate depends on the resistance offered by the microenvironment to the motion of the probe, fluorescence polarization provides an estimate of the environmental resistance which is interpretable as an apparent microviscosity and ultimately as a measure of fluidity62. In the present study no significant change in the fluidity parameters were observed in the BBM and the liposomes for the treatment groups. This indicates that aspirin, nimesulide and celecoxib at their respective doses dont affect the fluidity of the membrane as such.
References
1. Vane JR: Inhibition of prostaglandin synthesis as a mechanism of action for aspirin like drugs. Nat New Biology 1971; 231:232-235. [ Links ]
2. Vander FJ, Buytenhek M, Nugteren DH, Van Dorp DA: Acetylation of prostaglandin endoperoxide synthetase with acetylsalicylic acid. Eur J Biochem 1980; 109: 1-8. [ Links ]
3. Hamor GH: Non steroidal anti-inflammatory drugs. En: Principles of medical chemistry (Foye Wo. Ed) Vol. 15, pp. 30. Philadelphia, Lea & Febiger, 1989. [ Links ]
4. Bromm B, Rundshagen I, Scharein E: Central analgesic effects of acetylsalicylic acid in healthy men. Arznein Forsch 1991; 41: 1123-1129. [ Links ]
5. Reynold JEF: En: Matrindale the extra pharmacopoeia. 30th Edtn pp. 3-21. The Pharmaceutical Press (London), 1993. [ Links ]
6. Graham DY, Smith JL: Aspirin and stomach. Ann Intern Med 1986; 104:390-398. [ Links ]
7. Tijssen JGP: Low-dose and high-dose acetylsalicylic acid, with and without dipyridamole: a review of clinical trial results. Neurology 1998; 51 (Supl. 3): S15-S16. [ Links ]
8. Roderick PJ, Wilkes HC, Meade TW: The gastrointestinal toxicity of aspirin: an overview of randomized controlled trials. Br J Clin Pharmacol 1993; 35: 219-226. [ Links ]
9. Rossoni G, Berti F, Buschi LM, Della Bella D: New data concerning the anaphylactic and antihistaminic activity of nimesulide. Drugs 1993; 46 (Supl. 1): 22-28. [ Links ]
10. Ferreira SH: The role of interleukins and nitric oxide in the mediation of inflammatory pain and its control by peripheral analgesic. Drugs 1993; 46 (Supl. 1): 1-9. [ Links ]
11. Davis R, Brogden RN: Nimesulide; An update of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy. Drugs 1994; 48 (3): 431-454 . [ Links ]
12. Wober W: Comparative efficacy of nimesulde and diclofenac in patients with acute shoulder, and a meta analysis of controlled trials in extra articular rheumatism and osteoarthritis. Rheumatology 1999; 38 (Supl. 1): 33-38. [ Links ]
13. Bjarnason I: Effects of nimesulide and naproxen on the human gastrointestinal tract: a double blind crossover study. Rheumatology 1999; 38 (Supl. 1): 24-32. [ Links ]
14. Sirica AE, Lai GH, Endo K, Zhang Z, Yoon BI: Cyclooxygenase-2 and ERBB-2 in cholangiocarcinoma: potential therapeutic targets. Semin Liver Dis 2002; 22: 303–313. [ Links ]
15. Steinbach G, Lynch PM, Phillips RK et al.: The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000; 342: 1946-1952. [ Links ]
16. Harris RE, Alshafie GA, Abou-Issa H, Seibert K: Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res 2000; 60: 2101-2103. [ Links ]
17. Schmitz JC, Preiser H, Maestracci D, Ghosh BK, Cada JJ, Crane RK: Purification of human intestinal brush border membrane. Biochim Biophys Acta 1973; 323: 98-112. [ Links ]
18. Dahlqvist A: Method for the assay of intestinal disaccharidases. Analyt Biochem 1964; 7: 18-25. [ Links ]
19. Bergmeyer HU: Phophatase (phosohomonoesterase) determination in serum with p-nitrophenyl phosphate. En: Methods of enzymatic analysis (Bergmeyer H.V. Ed) p. 283, Acad Press, New York, 1963. [ Links ]
20. Wills ED: Mechanism of lipid peroxide formation in animal tissues. Biochem J 1966; 99: 667-676. [ Links ]
21. Ellman GL: Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82: 70-77. [ Links ]
22. Habig WH, Pabst MJ, Jakoby WS: Glutathione S transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 1974; 249: 7130-7139. [ Links ]
23. Massey V, Williums Jr CH: On the reaction mechanism of yeast glutathione reductase. J Biol Chem 1965; 240: 4470-4475. [ Links ]
24. Luck H: Catalase, methods of enzymatic analysis. En: Methods of enzymatic analysis (Bergmeyer H.U. Ed) pp. 885-893. Academic Press, New York. 1971. [ Links ]
25. Kono Y: Generation of superoxide radical during auto oxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 1978; 186: 189-195. [ Links ]
26. Folch J, Lees M, Sloane-Stanely GH: A simple method for the isolation and purification of total lipids from nervous tissue. J Biol Chem 1957; 226: 497-509. [ Links ]
27. Fringes CS, Dunn RT: A colorimetric method for determination of total serum lipids based on the sulfo-phospho-vanillin reaction. Am J Clinl Pathol 1970; 53 (1): 89-91. [ Links ]
28. Zlatkis A, Zak B, Boyle AJ: A new method for direct determination of serum cholesterol. J Lab Clin Med 1953; 41: 486-492. [ Links ]
29. Dubious M, Giles KA, Hamilton JK, Rebers PA, Smith F: Colorimetric method for determination of sugars and related substances. Anal Chem 1956; 28: 350-356. [ Links ]
30. Ames BN: Assay of inorganic phosphate, total phosphate and phosphatase. En: Method in Enzymology. Vol. VIII pp. 115-118. Acad Press, New York, 1966. [ Links ]
31. Warren L: The thiobarbituric acid assay of sialic acid. J Biol Chem 1959; 234: 1971-1976. [ Links ]
32. Lakshmi B, Balasubramanian KA: Lipid peroxidation of colonocytes membranes. Indian J Biochem Biophy 1995; 32: 89-93. [ Links ]
33. Schachter D, Shinitzky M: Fluorescence polarization studies of rat intestinal microvillus membranes. J Clin Invest 1977; 59: 536-548. [ Links ]
34. Shinitzky M, Barenholz Y: Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate. J Biol Chem 1974; 249: 2652-2657. [ Links ]
35. Pottel H, Van der Meer W, Herreman W: Correlation between the order parameter and the steady state fluorescence anisotropy of 1, 6 diphenyl-1, 3, 5- hexatriene and an evaluation of membrane fluidity. Biochim Biophys Acta 1983; 730: 181-186. [ Links ]
36. Demircan B, Celik G, Suleyman H, Akcay F: Effects of indomethacin, celecoxib and meloxicam on glutathione, malondialdehyde and myeloperoxidase in rat gastric tissue. The Pain Clinic 2005; 4: 383-388. [ Links ]
37. Martensson J, Jain A, Meister A: Glutathione is required for intestinal function. Proc Natl Acad Sci USA 1990; 87: 1715-1719. [ Links ]
38. Yoshikawa T, Naito Y, Kishi A, Tomii T, Kaneko T, Linuma S, Ichikawa H, Yasuda M, Takahashi S, Kondo M: Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut 1993; 34: 732-737. [ Links ]
39. Vaananen P M, Meddings J B, Wallace J L: Role of oxygenderived free radicals in indomethacin induced gastric injury. Am J Physiol 1991; 261: G470-G475. [ Links ]
40. Dormandy TL: Free-radical oxidation and antioxidants. Lancet 1978; 1: 647-650. [ Links ]
41. Ukawa H, Yamakuni H, Kato S, Takeuchi K: Effects of cyclooxygenase-2 selective and nitric oxide-releasing nonsteroidal antiinflammatory drugs on mucosal ulcerogenic and healing responses of the stomach. Dig Dis Sci 1998; 43: 2003-2011. [ Links ]
42. Sánchez S, Martín MJ, Ortiz P, Motilva V, Alarco N, De Lastra C: Effects of dipyrone on inflammatory infiltration and oxidative metabolism in gastric mucosa. Comparison with acetaminophen and diclofenac. Dig Dis Sci 2002; 47: 1389-1398. [ Links ]
43. Jiménez MD, Martín MJ, Pozo D, Alarco N, De Lastra C, Esteban J, Bruseghini L, Esteras A, Motilva V: Mechanisms involved in protection afforded by L-arginine in ibuprofen-induced gastric damage: role of nitric oxide and prostaglandins. Dig Dis Sci 2001; 47: 44-53. [ Links ]
44. Villegas I, Martín MJ, La Casa C, Motilva V, De La Lastra CA: Effects of oxicam inhibitors of cyclooxygenase on oxidative stress generation in rat gastric mucosa. A comparative study. Free Radic Res 2002; 36: 769-777. [ Links ]
45. Solimano G, Burgess EA, Levin B: Protein calorie malnutrition: effect of deficient diets on enzyme levels of jejunal muosa of rats. Br J Nutr 1967; 21: 55-68. [ Links ]
46. Troglia OM, Laughrey EG, Henley KS: Effect of quantitative undernutritiion on the activities of intestinal dissacharidases in the rat. Gastroenterology 1970; 58: 669-672. [ Links ]
47. Blair D, Yakimets W, Tuba J: Rat intestinal sucrase II. The effects of age, sex and diet on sucrase activity. Can J Biochem 1963; 41: 917-929. [ Links ]
48. Deren JJ, Broitman SA, Zamcheck N: Effect of diet upon intestinal disaccharidases and disaccharide absorption. J Clin Invest 1967; 46: 186-195. [ Links ]
49. Rosenweig NS, Herman RH: Dose response of jejunal sucrase and maltase activities of isocaloric high and low carbohydrate diets in man. Am J Clin Nutr 1970; 23: 1373-1377. [ Links ]
50. Yamada K, Goda T, Bustermante S, Kolodosky O: Different effects of starvation on activity of sucrase and lactase in rat jejunoileum. Am J Physiol 1983; 244: G 449-455. [ Links ]
51. Ozols A, Sheshukova T: Adaptation of the activity of membrane carbohydrases of chick small intestine to various carbohydrates. Comp Biochem Physiol 1984; 77 b: 635-637. [ Links ]
52. Mahmood A, Aggarwal N, Dudya PK, Sanyal SN, Mahmood R, Subrahmanyam D: Effect of a single oral dose of DDT on intestinal uptake of nutrients and brush border enzymes in protein calorie malnourished monkeys. J Environ Sci Health (B) 1981; 15: 143. [ Links ]
53. Singh H, Chugh JC, Shembesh AM, Ben-Musa AA, Mehta HC: Hepatotoxicity of high dose salicylates therapy in acute rheumatic fever. Ann Trop Paediatr 1992; 12 (1): 37-40. [ Links ]
54. Brasitus TA, Schacter D: Membrane lipids can modulate guanylate cyclase activity of rat intestinal microvillus membranes. Biochim Biophys Acta 1980; 630: 152-156. [ Links ]
55. Lucio M, Ferreira H, Lima JLFC, Matos C, Castroc B, Reis S, Influence of some anti-inflammatory drugs in membrane fluidity studied by fluorescence anisotropy measurements. Phys Chem Chem Phys 2004; 6: 1493-1498. [ Links ]
56. Matsumotu T, Fontaine O, Rosumusson H: Effect of 1, 25-dihydroxyvitamin D3 on phospholipid metabolism in chick duodenal mucosal cells. Relationship to its mechanism of action. J Biol Chem 1981; 256: 3354-3360. [ Links ]
57. Proulx P: Structure function relation in intestinal brush border membrane. Biochim Biophys Acta 1991; 1071: 255-271. [ Links ]
58. Aloni B, Shinitzky M, Livne A: Dynamics of erythrocyte llipids in intact cells, in ghost membranes and in liposomes. Biochim Biophys Acta 1974; 348: 438-447. [ Links ]
59. Shinitzky M, Inbar M: Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J Mol Biol 1974; 85: 603-615. [ Links ]
60. Shinitzky M, Inbar M: Microviscosity parameters and protein mobiliy in biological membranes. Biochim Biophys Acta 1976; 433: 133-149. [ Links ]
61. Weber G: Rotational Brownian motion and polarization of the fluorescence of solutions. Adv Protein Chem 1953; 8: 415-459. [ Links ]
62. Fuchs P, Parola P, Robbins W, Blout ER: Fluorescence polarizations and viscosities of membrane lipids of 3T3 cells. Proc Natl Acad Sci USA 1975; 72: 3351-3354. [ Links ]
![]() Address for correspondence:
Address for correspondence:
Dr. S. N. Sanyal.
Dept. of Biophysics
Panjab University. Chandigarth
India 160 014
E-mail: sanyalpu@gmail.com
Recibido: 10-III-2006.
Aceptado: 1-IV-2006.