Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.22 no.2 Madrid Mar./Abr. 2007
Recovery from experimental malnutrition with soymilk: immunological and genetic aspects
Recuperación de la malnutrición experimental con leche de soja: aspectos inmunológicos y genéticos
S. Fontenla de Petrino*, A. Prchal**, M. Fontenla*, A. M.ª Cena*, J. Gómez*, S. Pintos* and M.ª C. Peral*
*Cátedra de Biología. Departamento Biomédico. **Cátedra de Neurociencia. Departamento de Investigación. Facultad de Medicina. Universidad Nacional de Tucumán. R. Argentina.
ABSTRACT
Experimental malnutrition models have been useful to study the effects of malnutrition at early ages. Substantial evidence exists that malnutrition in critical stages of development could result in chromosomal damages. The effect of nutritional rehabilitation with soymilk as a complement of a restricted diet, on plasma and muscle proteins, chromosomal integrity, and unspecific and mucosa immune responses, was studied.
Adult male and female Wistar rats (5 weeks old) were assigned to different nutritional conditions: a) 14 days on protein restricted diet (corn flour and water), followed by 14 days in which water was replaced by soymilk, as nutritional rehabilitation; b) the same conditions above but periods of 28 days of a protein restricted diet, and 28 days of nutritional rehabilitation and c) age-matched malnourished (protein restricted diet without nutritional rehabilitation) and normally nourished controls.
After both nutritional rehabilitation periods, the weights reached were significantly higher (p < 0.001) than the malnourished control values, but lower than the normal control ones. Plasma protein concentrations were similar in all groups. Muscle proteins that were diminished during the restricted diet, reached normal control values after both rehabilitation periods. The protein restricted diet, produced numeric and structural chromosomal abnormalities. Nutritional rehabilitation was only partially able to revert these abnormalities. The phagocytic activity and gut mucosa IgA-secreting cells were significantly reduced (p < 0.001) during the restricted diet; both nutritional rehabilitation periods induced a significant increase of both, phagocytic activity and IgA secreting cells. These values were similar to controls.
Our results show that the supplementation of a protein-restricted diet with soymilk improved tissue protein content, as well as unspecific and gut mucosa immune responses, even though it was not able to reinstate fully normal body weight and a normal chromosome karyotype.
Key words: Malnourish. Soymilk. Chromosome abnormalities. Mucosa immunity. IgA.
RESUMEN
Los modelos de malnutrición experimental han sido útiles para estudiar los efectos de la misma en edades tempranas. Hay evidencia sustancial de que la malnutrición en etapas críticas del desarrollo podría producir daños cromosómicos. Se estudió el efecto de la rehabilitación nutricional con leche de soja como complemento de una dieta restringida sobre las proteínas plasmáticas y musculares, la integridad cromosómica y las respuestas inmunitarias inespecíficas de las mucosas.
Se asignaron ratas Wistar adultas macho y hembra (5 semanas de edad) a distintas condiciones nutritivas: a) 14 días con una dieta de restricción de proteínas (harina de maíz y agua), seguida de 14 días en los que se reemplazó el agua por leche de soja, como rehabilitación nutricional; b) las mismas condiciones anteriores pero con períodos de 28 días de dieta con restricción de proteínas y 28 días de rehabilitación nutricional; c) controles desnutridos (dieta de restricción proteica sin rehabilitación nutricional) y adecuadamente nutridos, emparejados por edad.
Tras ambos períodos de rehabilitación, los pesos alcanzados fueron significativamente superiores (p < 0,001) que las cifras en los controles desnutridos, pero inferiores a las de los controles normales. La concentración plasmática de proteínas fue similar en todos los grupos. Las proteínas plasmáticas, que estaban disminuidas durante la restricción dietética, alcanzaron los valores de los controles normales tras ambos períodos de rehabilitación. La dieta con restricción proteíca produjo anomalías en el número y estructura de los cromosomas. La rehabilitación nutricional sólo pudo revertir parcialmente estas anomalías. La actividad fagocítica de las células intestinales productoras de IgA estaba significativamente disminuida (p < 0,001) durante la dieta restringida; ambos períodos de rehabilitación nutricional produjeron aumentos significativos de ambos parámetros. Estos valores eran similares a los controles.
Nuestros resultados muestran que la complementación con leche de soja de una dieta restringida en proteínas mejoró el contenido tisular de proteínas así como las respuestas inmunitarias inespecíficas y de la mucosa intestinal, aunque no fue capaz de restablecer por completo a la normalidad el peso corporal y el cariotipo cromosómico.
Palabras clave: Desnutrido. Leche de soja. Anomalías cromosómicas. Inmunidad de la mucosa. IgA.
Background
Protein-energy malnutrition in children has been recognized as the most widespread nutritional problem around the world1. This disorder produces biochemical alterations leading to poor growth. The growth retardation varies in accordance with the severity and duration of the nutritional deficiency2.
Experimental malnutrition models have been useful to study the effects of malnutrition at early ages. Substantial evidence exists that malnutrition in critical stages of development could result in chromosomal damages3. Effects of starvation and marginal malnutrition on rat lymphocytes have been evaluated by chromosomal analysis before and after rehabilitation; reported results indicated that young animals exposed to conditions like starvation or chronic malnutrition are prone to permanent damage of the genetic system4. Cortés et al5 reported that malnutrition during lactation in rearing rats is associated with significantly increased DNA damage in spleen, peripheral blood, and bone marrow cells, as well as in isolated lymphocytes or lymphoid cells from the same tissues. This damage could be due to the deficiency of several essential nutrients required for protein synthesis that are associated with DNA integrity, impaired DNA repair mechanisms, and/or to the unavailability of molecules necessary to protect cells against DNA oxidative damage.
On the other hand, the increased level of DNA damage in peripheral blood lymphocytes and leukocytes could be related to negative effects, such as a deficient immune response, resulting in severe consequences, like relapse of previous infectious diseases or emergence of new ones. In fact, malnutrition results in reduced a number and functions of T-cells, phagocytic cells, and secretory immunoglobulin A antibody response. In addition, levels of many complement components are reduced6.
Protein malnutrition often coexists with deficiencies of specific micronutrients, such as zinc7, iron8, vitamin A9, folate10, and others, suggesting that the immune-depressing effects of protein deficiency may be the result of these associated micronutrient deficiencies.
An ideal food for the prevention and management of malnutrition should be of high nutritive value. Taking into account its nutritional profile11 and health effects12, soy could be considered as an ideal food for the prevention and management of malnutrition. In this line, we performed this study in order to determine the effect of the nutritional rehabilitation with soymilk, as a complement of a restricted diet, for as long as the malnutrition had lasted, on plasma and muscle protein levels, chromosomal abnormalities, and the unspecific and mucosa immune responses.
Material and methods
Animals
Male and female Wistar rats, supplied by the School of Medicine Central Animal Facilities (Universidad Nacional de Tucumán, Argentina), were used. Animals aged 5 weeks and weighing 85 + 5 g each, were housed in plastic cages, in a temperature-controlled room (21 + 1 ºC) with a controlled 12-hour light-darkness cycle (light on at 7:00 AM). Liquids and food of the different diets were available ad libitum. All experimental procedures were done in accordance with the relevant directives of the European Union (86/609/EEC) and the rules and recommendations of the FESSCAL (Federación de Sociedades Sudamericanas de la Ciencia de Animales de Laboratorio).
Experimental Design
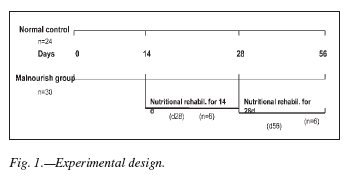
Animals were subject to a protein-restricted diet (corn flour and water), for two different periods of time, after which water was replaced by soymilk (AdeS, Unilever, Argentina) for nutritional rehabilitation. The first group (d28) received a 14 day period of nutritional rehabilitation after a similar time (14 days) of a protein-restricted diet. For the second group (d56) the periods of malnutrition and nutritional rehabilitation lasted 28 days each. Normally nourished and malnourished (without nutritional rehabilitation) age matched animals were used as controls (fig. 1).
On the 14th, 28th and 56th day, 6 rats from each control and experimental group were weighted and then anesthetized with ethylic ether and a sample of peripheral blood was taken. After that, the animals were killed by overexposure to ether. Then, the other samples such as peritoneal cells, quadriceps muscles and small intestine were taken (fig. 1).
The commercial soymilk used in this study (AdeS, Unilever, Argentina) is elaborated from a non-transgenic variety of soy. Its components are: protein: 2.6 g%; carbohydrates: 4 g%; fat: 1.5 g%, fiber: 0.5 g%; minerals: Ca, 11 mg% ; Fe 0.5 mg%; P, 40 mg%, Mg, 16 mg%; Na, 80 mg%; K, 150 mg%; vitamins: A, 60 mg/% ; B6, 0.2 mg%; B9, 20 mg%; B12, 0.1 mg%; D, 0.5 mg%, Isoflavones, 6.4 mg/% (daidzein: 2.4 mg% and genistein: 4 mg%).
Normally nourished rats received a commercial laboratory food (Cargill, Buenos Aires, Argentina). This commercial diet contains 24.6% of proteins from animal and vegetal origin.
Plasma and Muscle Protein concentration
Plasma protein concentration was determined by the Biuret method13 (Proti-2, Wiener Lab, Rosario, Argentina) using a control serum (Proti-2 Suero Patrón, Wiener Lab, Rosario, Argentina) as a standard.
In order to determine muscle protein, right hind limbs were removed and skinned. Quadriceps muscles were rapidly dissected, rinsed with saline solution to eliminate blood remnants, dried with tissue paper, and weigh. Tissues were homogenized in phosphate buffer pH 7 (w/v = 1/5). Then, 0.1 ml of Triton X-100 was added and mixed for 5 minutes. Finally, the homogenate was centrifuged at 20,000 rpm for 30 minutes. Proteins were determined in the supernatants by the Biuret method (Proti-2, Wiener Lab, Rosario, Argentina).
Results for both plasma and muscle protein concentrations are expressed in g%.
Cytogenetic analysis
In order to perform cytogenetic analysis, peripheral blood samples to which 1%, Sodium Heparin, 5,000 UI/ml (PL. Rivero y cia. SA, Industria Argentina) were obtained from the jugular vein and was added at the periods described above. Thirty drops of heparinized whole blood were added to each of two 15-ml polypropylene centrifuge tubes (Cellstar™, Greiner bioone, Germany) with 5 ml of RPMI 1640 culture medium (Gibco BRL, Grand Island, NY, USA) each, supplemented with 10% fetal calf serum (Gibco BRL, Grand Island, NY, USA), 2% glutamine (Gibco BRL, Grand Island, NY, USA), 1% phytohemagglutinin Mform (PHA-M) (Gibco BRL, Grand Island, NY, USA) and 0.1% gentamycin (Shering-Plough SA, Key Pharma S.A. Argentina) using a 21-gauge needle (Terumo, Japan). Cultures were incubated at 37 ºC for 72 h. At harvest time, to each culture 2 drops of Colcemid (Gibco BRL, Grand Island, NY, USA), were added, using a 25-gauge needle (Terumo, Japan), incubated for 30 more minutes at 37 ºC, and centrifuged for 8 minutes at 1,000 rpm. Then, the supernatant was removed, 4 ml of 0.075 M KCl were added to each pellet while kept in a 37ºC water bath, and resuspension performed. Immediately, cell suspensions were centrifuged for 8 min at 1,000 rpm, supernatants were removed, pellets were resuspended into 4 ml of fresh 0.075 M, KCl each, 5 drops of 3:1 methanol-acetic acid mix added in order to begin fixating the cells, centrifuged immediately for 8 min at 1,000 rpm, and resuspended into fresh 3:1 methanol-acetic acid mix. Pelleting and resuspension were repeated two more times. For making slides, 3-6 drops of cell suspension were poured along the upper long edge of a cold, wet slide, held in parallel to the lab counter with its surface at a 45º angle; the back and the bottom of the slide were dried immediately with tissue paper. Phase contrast microscopy (CH Olympus, Japan) was used to make sure that chromosomes were well spread and metaphases devoid of cytoplasm; if not, drying time were adjusted accordingly. Slides were placed in the oven at 95 ºC for 20 min for aging. Aged slides were immersed in 10% trypsin solution in 0.9% NaCl (Gibco BRL, Grand Island, NY, USA), for 50 seconds, and then in two baths of 0.9% NaCl solution; immediately, each slide was stained for 2 minutes with a fresh mix of pHydrion buffer (Micro Essential Laboratory Inc. U.S.A.) (3 ml) and 0.25% Wright’s stain (1 ml), (Sigma Chemical Co., St. Louis, MO, USA).
In order to determine the mitotic index, the total number of metaphases was counted in 100 nuclei and expressed as percentage.
The chromosomal abnormalities were analyzed in 50 G- banded metaphases per animal. Then, they were photographed, and their chromosomes counted on black and white prints. 3-5 of these metaphases were selected and karyotyped. Karyotypes were carried out according to nomenclature rules for rat G-banded chromosomes14.
Phagocytosis Test
The non-specific immune response was performed by an ex vivo phagocytosis test using peritoneal macrophages according to Perdigón et al., 198615. Peritoneal cells from rats of different groups and feeding periods were recovered. Macrophages were suspended at a concentration of 106 cells/ml in 5 ml of RPMI-1640 medium (Gibco BRL, Grand Island, NY, USA) supplemented with 0.3% (v/v) sodium heparin (PL. Rivero y cia. S.A., Industria Argentina). Then, 0.2 ml of Candida albicans suspension at a concentration of 5 x106 microorganisms/ml opsonized with rat autologous serum was added to 0.2 ml of each macrophage suspension and incubated for 15 min at 37 ºC. The reaction was stopped by icing. Immediately, smears were made, air dried, and stained with Giemsa (Biopack, Sistemas Analíticos SA, Buenos Aires, Argentina). The phagocytosis rate was measured by counting 100 cells from each of three smears per animal. The results were expressed as the mean + SD of the percentage of macrophages that had one or more yeast cells phagocyted.
Counting of IgA Secreting Cells by Immunofluorescence
As indicated above, samples from the small intestine were removed. The intestinal fluids were washed out with 0.01 M phosphate buffered saline solution, pH = 7.2. Tissues were placed in cold ethanol (96º) for processes with the Saint-Marie’s technique16. Once fixed, dehydrated and embedded in paraffin at 56 ºC, they were cut in 4 mm serial sections, and used to perform the immunofluorescence test in order to determine the number of IgA producing cells.
Histological samples from the small intestine were incubated with 0.2 ml of goat anti-rat IgA (a chain specific, Bethyl Inc., Montgomery, Texas, USA) at 1/20 dilution for 30 min, in a wet chamber at room temperature. Then, they were washed three times with 0.01 M phosphate buffered saline, pH = 7.2. A 1/100 dilution of the second antibody, fluorescein-conjugated rabbit F(AB’)2 fragment to goat IgG, whole molecule (Cappel, ICN Pharmaceuticals Inc., Costa Mesa, California, USA), was added and incubated for 30 min at room temperature. Afterward, the slides were rinsed again three times with the same buffer, air-dried, and mounted with 1:9 glycerol-buffer, pH 9.
Three histological sections from each animal and for each period were analyzed. The number of IgApositive cells within villi lamina propria was determined in 30 fields (magnification 40x). The results were expressed as the mean + SD of positive cells in the lamina propria per 10 villi.
Statistical Analysis
All statistical calculation was carried out with Sigma Stat software package (Jandel Scientific, San Raphael, California, USA). Differences between diet groups were analyzed by one way analysis of variance (ANOVA), and Dunnet’s test was used as a post-hoc test. Differences were considered significant if p < 0.05.
Results
Effect of Soymilk rehabilitation on body weight records
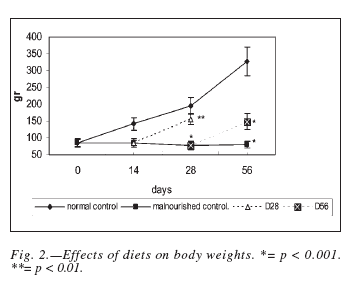
Figure 2 shows weight evolution during the experiment periods across different diet groups. At the beginning of the study, the mean body weight was 85 ± 5 g. A normally nourished animal regularly increased in body weight while malnourished does not. Weights reached, after both periods of nutritional rehabilitation were significantly superior (p < 0.001) to the malnourished control values, but significantly diminished when compared to age-matched normal control ones (d28: p < 0.01, d56: p < 0.001).
Effect of Soymilk rehabilitation on Plasma and Muscle Protein
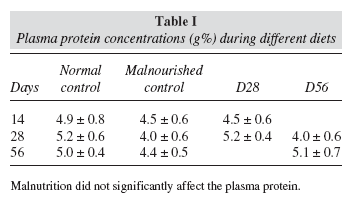
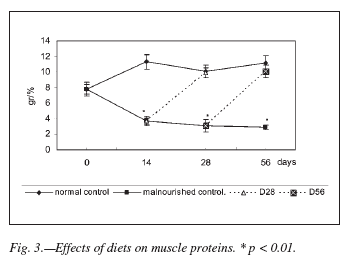
Plasma Protein concentration did not show significant differences between experimental and control groups at any data point (table I). However, muscle proteins tested were significantly decreased during the restricted diet (p < 0.01) in relation to the normal group. After both rehabilitation periods, muscle protein levels were similar to those showed by normal control groups. These results are detailed in figure 3.
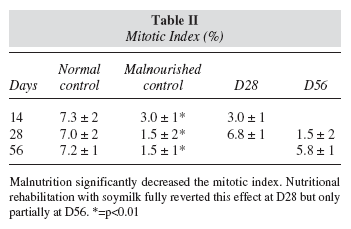
Effect of Soymilk rehabilitation on the mitotic index and on induced chromosomes abnormalities
During the restricted diet, the mitotic index was significantly lower in malnourished rats when compared with age-matched normal controls (p < 0.005). Nutritional rehabilitation with soymilk was able to restore normal values when malnutrition had lasted 14 days (d28), but not when it lasted 28 days (d56): in this case, the mitotic index of nutritionally rehabilitated animals was only 80-85% of that showed by normal control (table II).
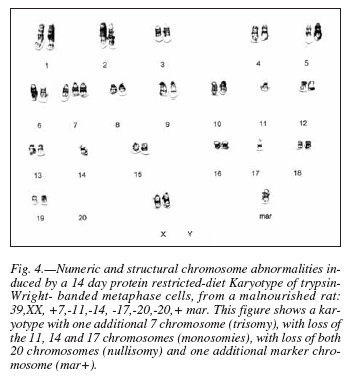
After 14 days of the restricted diet, numeric chromosomes abnormalities consisting of hypodiploid metaphases with 38-39 chromosomes, nullisomies (chromosome pair 20 lost) and monosomies (e.g.: one chromosome lost from pair 17, 14, and 11). Other numeric abnormalities like trisomies (e.g. pair 17) were also observed (fig. 4).
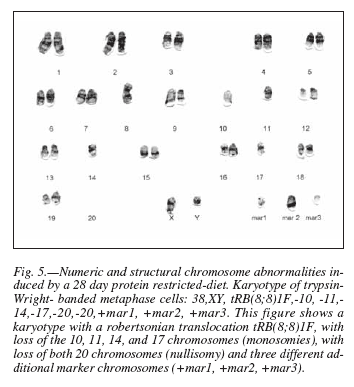
After 28 days of protein malnutrition, besides the numerical abnormalities, we observed structural anomalies like robertsonian translocations [T(8;8)]. Unidentified marker chromosomes were also observed in 80% of the metaphases analyzed (fig. 5). A robertsonian translocation is originated by a centric fusion of the long arms, of the acrocentric chromosomes, usually with simultaneous loss of both short arms15. A marker chromosome (mar) is a structurally abnormal chromosome in which no part can be identified by routine banding. In the description of karyotypes the presence of a mar must be preceded by a plus sign (+). When several different markers are clonally present, they may be indicated by an Arabic number after the symbol mar, e.g. mar 1, mar 2, etc. It must be stressed that this does not mean derivation of the marker from chromosome 1, chromosome 2, and so on17.
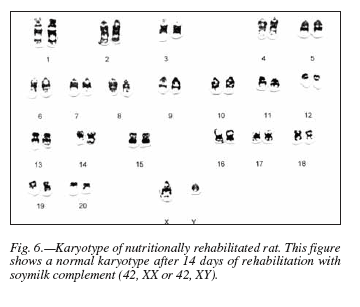
After 14 days rehabilitation with soymilk, a normal karyotype (42, XX or 42, XY) (fig. 6) was observed in 54% of the analyzed metaphases; however, animals that had to undergo rehabilitation for 28 d displayed only 16% chromosomally normal metaphases.
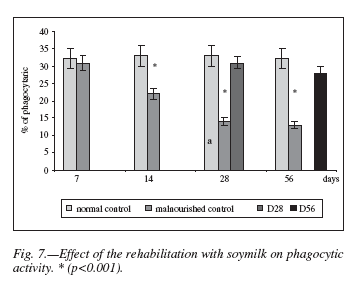
Effect of Soymilk on the Phagocytic Activity
Figure 7 shows the phagocytic activity of rat peritoneal macrophages in the different experimental groups. It can be seen that phagocytosis was significantly reduced (p < 0.001) in the restricted diet groups compared with the normal control. After both periods of soy milk rehabilitation, phagocytosis percentages incremented to values similar to those displayed by age-matched normal controls.
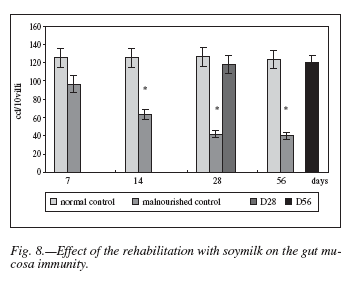
Effect of a Soymilk complement on the Number of IgA-secreting Cells
This assay was performed in order to determine the effect of the rehabilitation with soymilk as a complement of the restricted diet on the gut mucosa immunity.
In this case, results were similar to the phagocytic assays, i.e. numbers of IgA-positive cells were significantly reduced (p < 0.001) among malnourished animals when compared to normal control ones. The soymilk administered during both rehabilitation periods produced a remarkable increment in the number of IgA-producing cells in the lamina propria of villi of intestinal mucosa, reaching values similar to the normal ones (fig. 8).
Discussion
Malnutrition is multifactorial; however, the immediate cause of this condition is inadequate intake and poor utilization of nutrients due to inadequate food supply or poor quality foods18. There is considerable evidence that malnutrition has effects on physical growth, morbidity, mortality, cognitive development, reproduction, physical work capacity and risks for several adult onset chronic diseases19,20.
Soymilk, as well as a combination of soybeans with maize, has been found to be valuable in the management of malnutrition21.
In our study we used a rat model of chronic malnutrition, in which corn flour was the only solid food intake. This diet initially produced some weight loss, but its most remarkable effect in this regard was the absolute failure to gain weight (fig. 2). This result is similar to that reported by Qureshi et al22. Rehabilitation of malnourished animals by complementing the restricted diet with soymilk, allowed a significant but incomplete weight recovery (fig. 2).
Moreover, malnutrition produced a significant decrease of muscle but not plasma protein (fig 3 and table I). Similar findings have been reported in other studies that showed that there was a marked reduction in the rate of muscle proteins synthesis in rats fed a low protein diet23, and of proteins in muscle and other organs and tissues in rats fed a protein-free diet24. It was postulated that chronic protein deficiency leads to an overall reduction of protein synthesis that does not include certain plasma proteins. In others words, the protein metabolic response to the nutritional stress could be a redistribution of amino acids from the peripheral tissues to the liver for the synthesis of proteins that have a rapid turn over and are critical for survival25.
The nutritional quality of a protein food depends on content, digestion, absorption, and utilization of amino acids. Availability of amino acids varies with protein source, food processing treatment, and interaction with other components of the diet. Thus in the case of the diets used in our experiments it is important to note that corn proteins are of poor quality because of their low tryptophan and lysine content26. On the other hand, there are data showing that well-processed soyprotein isolates and soy-protein concentrates can serve as the major, or even sole, source of protein intake and that their protein value is essentially equivalent to that of food proteins of animal origin27. The soy milk we used (ADES) had all the essential amino acids. In this way it could be explained how nutritional rehabilitation with soy milk as a complement of corn flour, induced a tissue protein recovery to almost normal values (fig. 3).
Some authors3,28 have reported that malnutrition provokes chromosome anomalies, while others postulate the opposite29,30. In the present work, we detected abnormalities of the cell chromosome number after two weeks of malnutrition (fig. 4). Our data show that the longer period of the protein restricted diet, the more affected is the integrity of the chromosomes (fig. 5). Once chromosomal damage has occurred, nutritional rehabilitation with soymilk consistently has a limited effect in reverting it (fig. 6). This limitation is more noticeable when the length of the malnutrition period increases in spite of the fact that the rehabilitation period also increased. In this regard, our results agree with Alu and Murthy3. The lack of chromosomal abnormalities reported by others24,25 may be due to differences in the restricted diet and the techniques used for detecting them.
We have also observed that the restricted diet provokes a significant decrease in the lymphocyte mitotic index, which was reverted to a considerable degree when we complemented the restricted diet with soymilk (table II). This agrees with papers using other cell systems in experimental animals31,32. We think that this effect could be due mainly to the deficiency of essential amino acids and micronutrients that are necessary for the protein synthesis of mitotic spindles.
It is a well known fact that protein malnutrition induces a decrease in phagocytic activity of peritoneal macrophages33. Our results corroborate that the phagocytic function of macrophages is susceptible to protein malnutrition (fig. 7). It has been suggested that functional alterations of macrophages may be associated with micronutrient deficiencies, since protein restriction often coexists with them6,7.
IgA-secreting cells play an important role in the local immunological defense against pathogens and damage resulting from absorption of foreign antigens34. Also, dietary protein, minerals and vitamins are important in the mucosal IgA response of the small intestine5. Results reported here confirm this, since a significative decrease of IgA producing cells in the gut lamina propria was observed during the experimental malnutrition (fig. 8).
Early nutritional rehabilitation is needed to improve survival of malnourished individuals and to support immune responses8. Our results show that the rehabilitation with soymilk-complemented corn flour increased notably not only the peritoneal macrophage activity, but the mucosal immune function as well by stimulating the IgA-secreting cells (fig. 7 and 8). These data would suggest that high quality soy protein10 and/or micronutrients present in the soymilk administered like A, B12, B6, B9 vitamins and iron, may enhance or preserve immune function. The latter is supported by reports that the use of micronutrient supplements, singly or in combination, stimulates immune response and thus influences the susceptibility of a host to infectious diseases35.
The mechanisms by which the soymilk complement may exert immunomodulator effects have to be investigated. We hypothesize that the nutritive components of the soymilk could induce, within the lamina propria, T cells to produce Th2 IgA stimulating cytokines, which could modulate IgA production by B cells.
On the other hand, soy oligosaccharides (raffinose and stachyose) could have a prebiotic effect. Prebiotics are indigestible food ingredients that can beneficially affect the host by selectively stimulating the growth and/or activity of certain endogenous microbial population groups such as bifidobacteria and lactobacilli36. It has been reported that bifidobacteria enhances immune responses by increasing antibody production and proliferation of B cells in Peyer’s patches37.
In our experimental model, the supplementation of a restricted diet with soymilk, for a period of time identical to that of the previous malnutrition, normalizes tissue protein concentration, and appears to do the same with unspecific and gut mucosa immune responses; however the diet does not permit the recovery of fully normal body weight and the elimination of chromosomal abnormalities. We consider that a longer rehabilitation time would be necessary for complete recovery.
Acknowledgements
This work was carried out with the facilities of the School of Medicine at the National University of Tucumán, Argentina.
The authors thank UNILEVER (Argentina) for free supplying the AdeS soymilk used in this work. We also grateful thank Patricia Black-Decima, PhD for their helpful discussions during the preparation of the manuscript.
References
1. Food and Agriculture Organization of the United Nations: Undernourishment around the world. In: The state of food insecurity in the world 2004. Rome; 2004. [ Links ]
2. Muller O, Krawinkel MC: Malnutrition and health in developing countries. CMAJ 2005; 173: 279-286. [ Links ]
3. el-Ghazali S, Mikhail M, Awadallah M, Shatla H: Significant increase of chromosomal damage in protein energy malnutrition. J Trop Med Hyg 1990; 93: 372-376. [ Links ]
4. Alu V, Murthy PB: Chromosomal abnormalities in starved and marginally malnourished rats and in uterus upon rehabilitation. Experientia 1993; 49: 258-262. [ Links ]
5. Cortés E, González C, Betancourt M, Ortiz R: Assessment of DNA damage in spleen, bone marrow and peripheral blood from malnourished rats by single cell gel electrophoresis assay. Teratog Carcinog Mutagen 2001; 21: 231-247. [ Links ]
6. Chandra RK: Nutrition and immunology: from the clinic to cellular biology and back again. Proc Nutr Soc 1999; 58: 681-683. [ Links ]
7. Shankar AH, Prassad AS: Zinc and immune function the biological basis of altered resistance to infection. Am J Clin Nutr 1998; (Supl. 2): 447S-463S. [ Links ]
8. Openheimer SJ: Iron and its relation to immunity and infections disease: J Nutr 2000; (Supl. 2-2): 616S-633S. [ Links ]
9 Field CJ, Johnson IR, Schey PD: Nutrients and their role in host resistance to infection. J Leukoc Biol 2002; 711: 16-32. [ Links ]
10. Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM: Folate levels and neural tube defects. J Am Med Assoc 1995; 274: 1698-1702. [ Links ]
11. Young VR: Soy protein in relation to human protein and amino acid nutrition. J Am Diet Assoc 1991; 7: 828-835. [ Links ]
12. Messina MJ: Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr 1999; (Supl.3): 439-450. [ Links ]
13. Itzhaki RF, Gill DM: A micro-biuret method for estimating proteins. Anal Biochem 1964; 9: 401-410. [ Links ]
14. Levan G: Nomenclature rules for rat chromosome G-Bands. Hereditas 1974; 77: 37-52. [ Links ]
15. Perdigón G, Nader de Macías ME, Álvarez S, Oliver G, Pesce de Ruiz Holgado A: Effect of perorally administered lactobacilli on macrophage activation in mice. Infect Immun 1986; 53: 104-410. [ Links ]
16. Saint-Marie G: A paraffin embedding technique for studies employing immuno-fluorescence. J Histochem Cytochem 1962; 10: 250-256. [ Links ]
17. Mittelman F (Ed): ISCN 1995. An International System for Human Cytogenetic Nomenclature. Basel: S Karger Publisher; 1995. [ Links ]
18. King FS, Burgess A: Nutrition for Developing Countries (2nd Edition). London: ELBS with Oxford University Press; 1993. [ Links ]
19. Semba RD, Bloem MW (Eds): Nutrition and Health in Developing Countries: Humana Press 2001. [ Links ]
20. Martorell R, Rivera J, Lutter CK: Interaction of diet and disease on child growth. In Breastfeeding, Nutrition, Infection, and Infant Growth in Developed and Emerging Countries. Edited by Atkinson SA, Hanson LA, Chandra RK. Newfoundland: ARTS Biomedical Publishers and Distributors St. John's; 1990: 307-321. [ Links ]
21. Abiodun PO: Use of soya beans for the dietary prevention and management of malnutrition in Nigeria. Acta Paediatr Scand Suppl 1991; 374: 175-182. [ Links ]
22. Qureshi MI, Qureshi Z: Effect of protein malnutrition on the weight and serum albumin of albino rats. J Ayub Med Coll Abbottabad 2001; 13: 8-10. [ Links ]
23. Tawa NE Jr, Goldberg AL: Suppression of muscle protein turnover and amino acid degradation by dietary protein deficiency. Am J Physiol 1992; 263: 317-325. [ Links ]
24. McNurlan MA, Fern EB, Garlick PJ: Failure of leucine to stimulate protein synthesis in vivo. Biochem J 1982; 204: 831-838. [ Links ]
25. Jahoor F, Wykes L, Del Rosario M, Frazer M , Reeds P: Chronic Protein Undernutrition and an Acute Inflammatory Stimulus Elicit Different Protein Kinetic Responses in Plasma but Not in Muscle of Piglets. J Nutr 1999; 129: 693-699. [ Links ]
26. Friedman M, Brandon D: Nutritional and Health Benefits of Soy Proteins. J Agric Food Chem 2001; 3: 1069-1086. [ Links ]
27. Young VR: Soy protein in relation to human protein and amino acid nutrition. J Am Diet Assoc 1991; 7: 828-835. [ Links ]
28. Vijayalaxmi R: Chromosomal aberrations in malnutrition Metabolism 1975; 24: 1415-1417. [ Links ]
29. Khouri FP, McLaren DS: Cytogenetic studies in protein calorie malnutrition. Am J Hum Genet 1973; 25: 465-470. [ Links ]
30. Olmos S, García Reinoso F, Márquez MG, Roux, ME: Cytogenetic Studies in Bone Marrow Cells From Wistar Rats in Protein Malnutrition. Metabolism 2001; 50: 1025-1029. [ Links ]
31. Dunki J, Ruevekamp M, Hart GA, De Graaf, PW: Dietary influences on cell proliferation in bone marrow. Eur J Cancer Clin Oncol 1989; 25: 953-957. [ Links ]
32. Mengheri E, Nobili F, Crocchioni G, Lewis JA: Protein starvation impairs the ability of activated lymphocytes to produce Interferon-Gamma. J Interferon Res 1992; 12: 17-21. [ Links ]
33. Teshima S, Rokutan K, Takahashi M, Nikawa T, Kido Y, Kishi K: Alteration of the respiratory burst and phagocytosis of macrophages under protein malnutrition. J Nutr Sci Vitaminol (Tokyo) 1995; 41: 127-137. [ Links ]
34. Mestecky Y , Mcghee JR: Prospect for human mucosal vaccines. Adv Exp Med Biol 1992; 327: 13-23. [ Links ]
35. Bhaskaram P: Micronutrient malnutrition, infection, and immunity: an overview. Nutr Rev 2002; 60: 40-45. [ Links ]
36. Gibson GR, Roberfroid MB: Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 1995; 125: 1401-1412. [ Links ]
37. Yasui H & Ohwaki M: Enhancement of Immune response in Peyer's Patch cells cultured with Bifidobacterium breve. J Dairy Sci 1991; 74: 1187-1195. [ Links ]
![]() Correspondence:
Correspondence:
S. Fontenla de Petrino
José Colombres, 255
4000 S. M. de Tucumán - R. Argentina
E-mail: fontenla@arnet.com.ar
cbiologia@fm.unt.edu.ar
Recibido: 23-XII-2005.
Aceptado: 19-V-2006.