Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.22 no.5 Madrid sep./oct. 2007
Intestinal toxicity of non-steroideal anti-inflammatory drugs with differential cyclooxigenase inhibition selectivity
Toxicidad intestinal de los fármacos antiinflamatorios no esteroideos con una selectividad diferenciada en la inhibición de la ciclooxigenasa
S. Chopra, R. Kishore Saini and S. Nath Sanyal*
Department of Biophyisics. Panjab University. Chandigarth-160014. India.
ABSTRACT
The present study was designed to investigate the gastrointestinal side effects of cycloxygenase (COX) inhibitor with varying selectivity, called the non-steroidal antiinflammatory drugs (NSAIDs) viz., non-selective COX-1 & 2 inhibitor -aspirin, prefentially selective COX-2 inhibitor- nimesulide and highly selective COX-2 inhibitor- celecoxib. Treatment with NSAIDs exhibited a decrease in the activity of rat intestinal brush border membrane associated enzymes such as sucrase, lactase, maltase and alkaline phosphatase as compared to the control in the duodenum, jejunum and ileum. The uptake of D-glucose and L-histidine in the everted intestinal sac was found to be decreased. Also the decease of glucose and histidine uptake was found to be dependent on the substrate concentration, temperature and the time interval of incubation. The physical state and composition of brush border membrane was found to be altered as evident in the FTIR spectrum, by appearance of new peaks while disappearance of certain peaks occurred which were characteristics of the control membrane. The changes in wave number as well as peaks height were also noticed. Alterations in protein profile of the membrane were demonstrated using SDS-PAGE analysis where disappearance of few bands and change in the relative intensities of the bands were noticed and correlated with the alterations that have taken place at the molecular level. Histological studies have depicted a marked decrease in the absorption surface area such as the villi height of the intestinal segment. In addition, crypt number also deceased in the treated animals, an indication that such changes also correlate well with the changes in the transport of the end product nutrients.
Key words: Non-steroidal anti-inflammatory drugs. Rat intestinal membrane. Structure and function.
RESUMEN
Se diseñó este estudio para investigar los efectos adversos gastrointestinales de los inhibidores de la ciclooxigenasa (COX) con selectividad variable, denominados fármacos antiinflamatorios no esteroideos (AINE), inhibidores no selectivos de la COX-1 y la COX-2 -aspirina, los inhibidores predominantemente selectivos de la COX-2- nimesulida, y los inhibidores muy selectivos de la COX-2 -celecoxib. El tratamiento con AINE mostró un descenso de la actividad de las enzimas asociadas a la membrana en cepillo intestinal de la rata, tales como sucrasa, lactasa, maltasa y fosfatasa alcalina, en comparación con el control, en el duodeno, yeyuno e íleon. Se halló que la captación de D-glucosa y L-histidina en el saco intestinal revertido estaba disminuida. También se encontró que la captación de glucosa e histidina dependía de la concentración de sustrato, la temperatura y el intervalo de tiempo de incubación. El estado físico y la composición de la membrana en borde en cepillo estaban alterados como se evidenció en el espectro FTIR, con aparición de nuevas picos y desaparición de ciertos picos característicos de la membrana control. También se apreciaron cambios en el número de ondas así como la altura de los picos. Se demostraron cambios en el perfil de proteínas de membrana mediante el análisis SDSPAGE, apreciándose desaparición de algunas pocas bandas y cambio en las intensidades relativas de las bandas, correlacionándose con las alteraciones que ocurrían a nivel molecular. Los estudios histológicos han mostrado un descenso marcado de la superficie de absorción, como por ejemplo la altura de las vellosidades del segmento intestinal. Además, el número de criptas también disminuyó en los animales tratados, un indicador de que tales cambios también se correlacionan bien con los cambios en el transporte de los nutrientes finales.
Palabras clave: Fármacos antiinflamatorios no esteroideos. Membrana intestinal de la rata. Estructura y función.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are the most common class of over the counter medication used world wide.1 NSAIDs range from the classic "miracle drug" aspirin to the latest creation "super aspirin" commonly known as coxibs.2 The therapeutic benefits of NSAIDs are alleviation of pain, inflammation and fever.3 They act by inhibiting the cycloxygenease (COX) enzymes which form prostaglandins from the arachidonic acids.4 Recently, two isoforms of cycloxygenase have been identified; COX-1 is believed to have gestroprotective role, while COX-2 is responsible for the production of proinflammatory mediators. 5 The various NSAIDs can be classified depending on the selectivity to inhibit one isoform of COX over the other. Different NSAIDs vary in their relative COX-1 and COX-2 specific selectivity.6 The capacity of any NSAID to inhibit prostaglandin production by these enzymes is expressed as the inhibitory concentration of 50% of an enzyme (IC50). The ratio of IC50 of COX-2 to COX-1 defines the COX selectivity. The non-selective inhibition of COX isoform by traditional NSAIDs (salicylates) is responsible for various toxic effects, especially the gastric ulceration and bleeding, inhibition of renal blood flow, inhibition of platelet aggregration and gastrointestinal perforations or obstruction.7
The COX-2 inhibitors (coxibs) are the latest addition of the growing armamentarium of the anti-inflammatory drugs.8 Their ability to selectivly block the formation of pro-inflammatory prostaglandins while sparing those that guard the gastrointestinal tract makes them an attractive choice for long term use.9 Since COX-2 inhibitors have a theoretically more favorable side effect profile than traditional NSAIDs, the present study has been undertaken to compare the intestinal toxicity and tolerability of classic non-selective NSAID (aspirin), a preferentially COX-2 selective (nimesulide) and a highly COX-2 selective NSAID (celecoxib). The investigation has been conducted on the structure and function of rat intestinal segment, the uptake of endproduct nutrients like D-glucose and L-histidine, the analysis of the intestinal brush border membrane intrinsic proteins, enzymatic activities of the intestinal marker enzymes, analysis of the functional groups in the membrane by Fourier Transform Infra Red Spectroscopy (FTIR) and qualitative and quantitative histological changes by morphometric analysis.
Material and methods
Animal and drug treatment
Wistar rats weighing between 150-175 g were taken for the experimental procedure from the central animal house of the Panjab University. They were housed in the polypropylene cages embedded with rice husks and maintained on rat pellet chow and also had free access to water.
After acclimatization for one week, the animals were divided into four groups; Control (vehicle treated, physiological saline), aspirin, nimesulide and celecoxib, each 40 mg/kg body weight given orally. The drug treatment was continued for 28 days and on day 29, the animals after overnight fasting were given an overdose of ether anesthesia and sacrificed by rapid decapitation. In order to avoid diurnal variation in the parameters studied, the animals were sacrificed uniformly around 8.00 AM throughout the study.
From each animal, the intestine was removed, washed by flushing with ice-cold physiological saline, divided into duodenum, jejunum and ileum segments, weighed and proceeded for the analyses reported here.
Preparation of brush border membrane (BBM)
BBM from the intestinal segments was purified following the procedure of Schmitz et al.10 A known weight of each portion of the intestine was cut into small pieces in chilled 1 mM tris-50 mM mannitol buffer (pH 7.4) and homogenized in a motor driven homogenizer at 4 ºC. The resultant 10% homogenate was passed through two layers of cheese cloth and to the filtrate, anhydrous dry CaCl2 added slowly with continued stirring on a magnetic stirrer, to a final concentration of 10 mM and left for 10 min in cold. It was centrifuged at 2,000 g for 10 min at 4 ºC. Pellet obtained was discarded while the supernatant was centrifuged at 42,000 g for 20 min. The supernatant obtained ftom the later spin was discarded while the pellet resuspended in the same buffer which also contained 0.02% sodium azide (NaN3). The BBM prepared as above was essentially free from mitochondria, microsomes, lysosomes, basolateral membrane and others as assessed by the estimation of the marker enzymes.
Enzyme assays
The activity of the BBM-associated disaccharidases, such as sucrase, lactase and maltase were determined by measuring the D-glucose liberated from the respective disaccharide substrate and using a glucose oxidase-peroxidase enzymatic system of Dahlqvist,11 where the resultant pink color was being measured at 560 nm.
The activity of alkaline phosphatase was assayed according to the method of Bergmeyer,12 where pnitrophenyl phosphate was used as the substrate which was hydrolyzed by the enzyme to yield p-nitrophenol to be measured at 410 nm.
Protein concentration in the membrane was determined by the method of Lowry et al.13 using bovine serum albumin as the standard and the optical density measured at 660 nm.
Transport of glucose and histidine in jejunum
Preparation of intestinal sacs. After dissecting the rat, the abdomen was opened by a midline incision, jejunum removed and flushed with physiological saline for cleaning. The everted sac was prepared by inserting a narrow glass rod with thickening at one end into the lumen and eversion of the gut is done carefully by reaching to the end and folded over it carefully and then pushing the rod through the evrted end of the emerging intestinal segment. One end of the everted sac was ligated carefully, glass rod removed and the sac placed in the saline solution. A syringe filled with Kreb's Ringer phosphate buffer (KRP)- glucose was inserted into the sac in a small vol. The syringe was removed and the sac once again tied with the thread at the open end. Care was taken to ligate the sacs at both ends firm and tight enough to prevent any leakage but not too tight to damage the tissue. The method is based on the work of Mizuma et al.14
L-histidine uptake. The sacs were immersed in a solution of KRP in small reaction vessels in the presence of different concentrations of histidine and incubated for different time interval and temp. The vessels were put in shaking water bath and at the end of the incubation the sacs were removed, exterior washed, punctured with a needle and the contents taken in test tubes to which a weak acetic acid solution (0.35 mol/L) added to deproteinize the solution. The tubes were covered with aluminium foil and placed in boiling water bath for 10 min. After cooling, an aliquot of the deproteinized solution taken and to it added sodium nitrite (50 g/L prepared fresh). The tubes were left for 5 min with occasional stiring and added sodium carbonate solution (75 g/L) with vigorus shaking. To it was added ethanol and distilled water, mixed thoroughly and after 30 min the OD read at 498 nm. The standard used was L-histidine at a concentration of 0.15 mol/L. The method was based on as described by Plummer.15
D-glucose uptake. To the deproteinized solution as above after incubation in the presence of KRP glucose and after centrifugation to obtaine a clear supernatant, the glucose oxidase-peroxidase enzyme system (GODPOD) was added11. The OD was taken at 590 nm after 30 min of incubation at room temp using different concentrations of D-glucose as standard.
Polyacrylamide gel electrophoresis
Membrane proteins were separated and analyzed by SDS-PAGE system following the method of Laemmli16 in a 10% separating gel and 3% stacking gel using a discontinuous buffer system. The protein sample and the SDS sample buffer (100 mM tris HCl, pH 6.8, containing 2% SDS, 0.15% bromophonel blue, 10% glycerol and beta-mercaptoethnol) were mixed in the ratio of 1:6, incubated at 37 ºC for 1 hr, centrifuged at 3,000 g for 15 min and the clear supernatant containing 60 μg of protein loaded into each well and electrophoresed at 100 volts. The protein bands were visualized by staining with Coomassi brilliant blue R-250 and the excess stain being removed in the destaining solution of acetic acid -methanol- water for 6-8 hr with frequent change. The gels were photographed in a gel documentation system image analyser with the appropriate software as provided by the company.
FTIR studies
A known amount of membrane preparation was washed with different grades of alcohol till the absolute alcohol and then mixed with KBr in the ratio of 5:95. The KBr powder taken was of spectroscopic grade and absolutely dry. The mixture was grounded in a homogeneous dispersion and processed to form a pellet at a pressure of 10-15 tonnes with the help of a hydraulic pressure machine. The pellet was transferred to the spectrophotometer sample holder and the FTIR spectra recorded in the range of 450-4,000 cm-1.17
Histological studies
Small pieces of intestinal segments were washed in ice cold saline and fixed in Bouin's fixative (picric acid, glacial acetic acid and formaldehyde) for 24 hr. After fixation, the tissues were carefully processed in alcohol, xylene and embedded in paraffin wax (58-60 ºC). Paraffin sections were cut at 5-7 μm thickness and then subjected to Delafield haematoxylin -eosin histological staining technique.18
Statistical analysis
The data was subjected to Student's "t" test and a "p" value of at least 0.05 or less than that was considered significant.
Result and discussion
BBM associated enzymes: Table I shows the results of the effects of an oral administration of aspirin, nimesulide, and celecoxib on the various intestinal marker enzyme in the BBM isolated from duodenum, jejunum and ileum segments of rat intestine. The administration of NSAIDs resulted in a significant decrease in general, in the activity of sucrase, lactase, maltase and alkaline phosphatase in the different intestinal tissue. Such decreased enzyme activity might have resulted due to either the reduced substrate affinity (kinetic effect) or modulation of protein molecular number or activity (metabolic effect), which needs further experimentation to elucidate the mechanism. In the treatment of natural steroids, Gray and Greenwood, 1982 have speculated that the reduction in the digestive enzyme activities might be due to the decreased food intake and hence, decreased protein content.19 Moreover, it is well documented that components of diet present in the intestinal lumen have a direct effect on the activities of BBM enzymes.20, 21 Kinetic evidence too suggests a close functional link between the carrier mediated sugar transport system and the disaccharide hydrolase in the intestine.22 The alkaline phosphatase activity is also coupled to the phosphate transport system.23
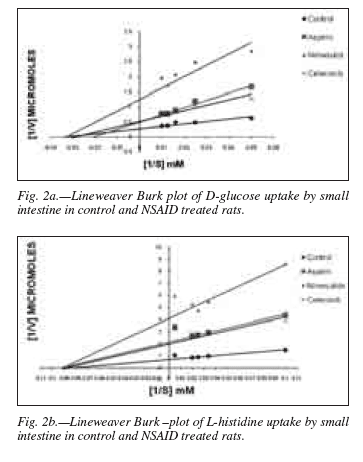
Uptake of D-glucose and L-histidine: The uptake studies of the end product nutrients of digestion such as glucose and histidine was carried out and the results show that the administration of NSAIDs produced a reduction in the uptake, hence, the transport of these metabolites in the intestinal segment (figs.1a,b). The movement of the substrate molecules, particularly across the BBM of the epithelial absorptive cells depends upon the carrier molecules (transport proteins). 24 Such mediated transport depends on the coupled movement of Na+ across the membrane using the steep concentration gradient of the ion and thereby called the "secondary active transport system".25 Thus, the decrease in the uptake of nutrients after NSAID treatment could be attributed to the changes either in maximum absorption affinity of the transport protein (km) or in the maximum uptake velocity (Vmax). To probe into such possibilities, experiment was carried out at different substrate concentrations and study the Michaelis-Menten kinetic parameters of the uptake of glucose and histidine. Lineweaver-Burk plot obtained of the double reciprocal of substrate (nutrient) and uptake velocity clearly demonstrats a decrease in the Vmax values of the treated animals with respect to the control (figs. 2a, b). The Km of the uptake of histidine did not show any change while the glucose uptake exhibited only a marginal increase (table II), thereby supporting the contention that the NSAID treatment does not significantly contribute to the alteration in the binding and transport of nutrient but may actually reduce the process by metabolically deactivating the protein. Ducis and Kaepsell (1983) however, concentrated on the lipid compositions required for optimum sodium dependent D-glucose transport in an experiment in reconstituted liposomes and concluded that in addition to cholesterol and phosphatidyl serine, the presence of phosphatidyl ethanolamine and sphingomyeline enhanced the transport activity further.26 Moreover, studies have suggested that alteration of membrane fluidity may influence the uptake of sodium dependent D-glucose transport in rat small intestine.27 The decrease in transport can also be attributed to the decease in cell number or ability of the NSAIDs to suppress the absorptive capacity of the enterocytes by suppression of specific carrier proteins. The effect of different temperature on the D-glucose and L-histidine transport has been studied and the data had been transformed to the Arrhenius plots of log 10 velocity of transport versus the reciprocal of absolute temperature. The transition temp (Tc) was directly taken from such plot while the energy of activation (Ea) was calculated from the slope of the Arrhenius line and presented in the table III.
The non-linearity of the Arrhenius plots indicates that the proteins involved in the transport process experience temperature induced changes in the membranes. 28 However, the discontinuity in the Arrhenius plots of both the control and the treated membrane was also observed at the identical temperature which rules out the possibility of any effective denatutation of the transport proteins between the fluid and ordered lipid domain of the membranes.29 There was no change in the transition temperature observed which evades any chance of the NSAIDs binding to the membrane lipid bilayer and possibly alter the phase transition or melting of the membrane lipids.30 In addition, the changes in the activation energy following the NSAIDs treatments reflect the significant alterations in the energy requirement of the carrier proteins for the binding of the substrate molecules.
D-glucose and L-histidine uptake was also studied at different time intervals which showed an initial rapid burst of both the nutrients and then showed down considerably after about 60 min of incubation, thereby reaching the equilibrium (figs. 3a, b). The saturation at longer time duration shows that the transport protein involved in the uptake become fully saturated with the substrate and no unoccupied binding sites left. The effect of aspirin and phenylbutazone on the intestinal absorption of glucose in vitro has been studied where incubation of the rat everted intestine in a modified Kreb's bicarbonate solution containing either aspirin or phenylbutazone resulted in an inhibition of the active glucose transport.31
SDS-PAGE of membrane protein: SDS-PAGE analysis was carried out with BBM associated protein samples under reducing conditions. An overnight staining with Commasie blue revealed the major protein bands in the molecular weight range of 14-97 kDa (fig. 4). The BBM of the control animals showed the presence of 8 major bands among which the proteins of the molecular weight 68, 43 and 29 kDa were the most abundant one. The aspirin, nimesulide, and celecoxib treatment demonstrated the presence of 5, 6 and 7 major bands, respectively. The observed changes in the number of protein bands in the NSAIDs treated groups as compared to the control can be related with the alteration that might have taken place at the molecular level such as that of the gene transcription, as recently there had been reports of the increased expression of NSAIDs activated gene (NAG-1) in colorectal cancer, oral cavity cancer and cancer of other tissues which had been associated with cycloxygenase inhibition and induction of apoptosis.32.
FTIR-spectroscopy of BBM: FTIR has proved to be particularly useful as a physicochemical technique to study the structure and organization of biomolecules,33 and therefore employed here to probe the dynamics of the BBM. FTIR spectra of BBM of different intestinal segments have been shown in figures 5 a-d and their physical characteristics summarized in table IV. Administration of aspirin, nimesulide and celecoxib caused the appearance of a few new peaks while same peaks already present in the control, have disappeared. Moreover, changes in the peak heights and a shift in the wave number has also been observed.
The secondary structure of the proteins can be distinguished by using a combination of amide I frequency and the intensity of amide II and III region.33 The peaks in the range of 1,650-1,653 cm-1 corresponding to α-helices and near 1,632 cm-1 correspond to β-sheets while near 1,655 cm-1 represent to the disordered state. The present data show a marked shift in the wave number from α-helical region towards the disordered state in jejunum BBM of all the treatment groups and also in the duodenal BBM of aspirin and celecoxib groups. This conformational change of protein can finally lead to the changes in the biological functions. From the shift in the wave number that has taken place in the jejunal segment of the intestine in particular, it can possibly be predicted that the NSAID treatment might have altered the transmembrane helical domains of various transporter proteins.
The complex amide II bands in the range of 1,480-1,575 cm-1 are difficult to differentiate for membrane proteins since the frequency differences are small or non-existent between various structural components. The amide III band arising in the region of 1,330-1,200 cm-1 has a broader range of absorbance, and therefore the bands associated with the various structures are better separated. The absorbances arising between 1,295 and 1,260 cm-1 are due to helical structure, bands between 1,245 and 1,230 cm-1 are attributed to β-sheet structure, and fairly broad medium- intensity bands from 1,260 and 1,240 cm-1 arise from the random conformation. The present data showed that most of the amide III bands in all segments of control and treated groups have α-helical structure. Apart from the shift in wave number, notable alteration in the peak heights has been depicted which may correspond to the changes in the lipid bilayer and hence are of great biological importance because it can lead to abnormal cellular function.
Histological analysis: The morphological changes induced in the different segments of the intestine following NSAID administration can be measured by qualitative and quantitative histological analysis. The changes that take place in the abnormal surface can be correlated well with the changes in the absorption and transport of various nutrients. The table V shows the results in villi number, villi height and crypt number in various intestinal segements upon treatment with the NSAIDs. A fairly significant decrease in villi number and villi height of the treated group was noted where the crypt number did not register any significant change.
Figure 6 a-d shows the disruption in the columner cell lining along with the shrinkage of lamina propria and shrinking of the villi. The crypts of Liberkühn were highly disorganized in the treated group. Along with the disrupted columnar cell lining, the broken tips of the villi cells were also observed. The decrease that is observed in the villi number, villi height and the disorganization of the villi surfaces may be correlated well with the decrease in absorption and transport of various nutrients following the NSAID treatment. Such changes are similar to the earlier findings as observed in different pathophysiological states.34
References
1. Vigdahl JK, Turkey RH. Mechanism of action of novel antiinflammatory drugs, diflumidone and R-805. Biochem Pharmacol 1977; 26:307-11. [ Links ]
2. Silverstein FE, Faich G, Goldstein JL. NSAIDs for osteoarthritis and rhemutoid arthritis. The class study: a randomized controlled trial. J Am Med Assoc 2000; 284:1247-55. [ Links ]
3. Agarwal NM. Epiedmiology and prevention of non-steroidal anti-inflammatory drug effects in the gastrointestinal tract. Br J Rhematol 1995; 34:5-10. [ Links ]
4. Dewitt DL. COX-2 selective inhibitor: the new super Aspirins. Drug Metab Despos 1999; 55 (4): 635-7. [ Links ]
5. Dubois RN, Abramson SB, Crofford L, Gupta RA. Cyclooxygenase in biology and disease, FASEB J 1998; 12:1063-73. [ Links ]
6. Hawkey CJ. COX-2 inhibitors. Lancet 1999; 353 (9149):307-14. [ Links ]
7. Hawkey CJ. Non-steroidal anti-inflammatory drug gastropathy: cause and treatment. Scand J Gastroenterol 1996; 220:124-7. [ Links ]
8. Wallace JL. Non-steroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology 1997; 112: 1000-16. [ Links ]
9. Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, specific cycloxygenase-2 inhibitor against colon carciongenesis. Cancer Res 1998; 58:409-12. [ Links ]
10. Schmitz JC, Preiscr H, Maestracei D, Ghosh BK, Cerda JJ, Crane RK. Purification of the human intestinal brush border membrane. Biochim Biophys Acta 1973; 323-98. [ Links ]
11. Dahlquist A. Methods for the assay of intestinal disaccharidases. Anal Chem 1964; 7:18-25. [ Links ]
12. Bergmeyer HU. Phosphatase (phosphomonoesterase) determination in serum with p-nitrophenyl phosphate. In: Methods of Enzymatic Analysis. (Ed. H. V. Bergmeyer) Acad. Press, New York, P 1973; 783. [ Links ]
13. Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem 1951; 193:265. [ Links ]
14. Mizuma T, Ohta K, Hayashi M, Awnzu S. Intestinal active absorption of sugar conjugated components by glucose transport system: implication of improvement of poorly absorbable drug. Biochem Pharmacol 1992; 43:2037-9. [ Links ]
15. Plummer DT. The transport of amino acids acrose the smell intestine. In: Plunner DT (Ed) . An Introduction to Practical Biochemistry. Tata McGrow Hill Co. New Delhi, pp 1988; 259-63. [ Links ]
16. Laemmli UK. Change of structural protein during the assemble of the head of bacteriophage T4. Nature 1970; 227:680. [ Links ]
17. Cortijo M, Chapman D. FEBS Lett 1981; 131: 245-8. [ Links ]
18. Pearse AGE. In: Histochemistry , theoretical and applied, 3rd Edn. Vol. 1. Churchill Livingstone (London) 1968; 660 p. [ Links ]
19. Gray JM, Green Wood MRC. Time course of effects of ovarian hormones on food intake and metabolism. Am J Physiol 1982; 243:E407. [ Links ]
20. Deren JJ, Broitman SA, Zamcheek N. Effect of diet upon intestinal disaccharidase and disaccharide absorption. J Clin Invest 1967; 46:189. [ Links ]
21. Goda T, Yamada K, Bustmante S, Kolodusky O. Dietary induced rapid decrease of microvilli carbohydrate activity in rat jejunum lumen. Am J Physiol 1983; 245:G418. [ Links ]
22. Ramasvamy K, Malatti P, Crane RK. Demonstration of hydrolase related glucose transport in brush border membrane vesicles prepared from guinea pig small intestine. Biochem Biophys Res Commun 1976; 68:162. [ Links ]
23. Petitclaere C, Plante GE. Can J Physiol 1981; 59:311. [ Links ]
24. Eilam Y, Stein WD. Kinetic studies of transport across red blood cell membrane. In: Methods in Membrane Biology, (ED. Kom, ed). Plenum Publishing Corporation, New York, Vol. 2 1974; 283 p. [ Links ]
25. Boyd CAR, Lund EK. L-proline transport by brush border membrane vesicles prepared from human placenta. J Physiol 1981; 315:9-19. [ Links ]
26. Ducis I, Kaepsell H. A simple liposomal system to reconstitute and assay Na+/ D-glucose co transport for kidney brush border membrane. Biochim Biophys Acta 1983; 730:119-29. [ Links ]
27. Brasitus TA, Dudeja PK, Dahiya R. Premalignant alteration in the lipid composition and fluidity of colonic brush border membrane of rats administered with 1,2-dimethyl hydrazine. J Clin Invest 1986; 77: 831-40. [ Links ]
28. Brasitns TA, Schacter D. Lipid dynamics and lipid protein interaction in rat enterocyte basolateral and microvillous membranes. Biochemistry 1980; 19: 2736-69. [ Links ]
29. Sanyal SN. Stmulation of uridine diphosphate galactose: ceramide galactosyl transferase by certain soluble protein factor in rat brain cytosol. Indian J Exp Biol 1987; 25:606-12. [ Links ]
30. Kaushal N, Sanyal SN. Alteration in L-histidine transport in response to aspirin and mimesulide induced toxicity in rat intestine using everted intestinal sacs. Toxicol Mech Methods 2006; 16:379-84. [ Links ]
31. Salch S, Khayyal MT, Masri AM El, Ghazal AM. Effect of acetyl salicylic acid and phenyl butazone on glucose absorption in vitro. Metabolism 1969; 18(7):599-605. [ Links ]
32. Kim KS, Back JS, Flake GP, Loftin CD, Crlvo BF, Elmig TE. Expression and regulation of non-steroidal anti-inflammatory drug activated gene (NAG-1) in human and mouse tissue. Gastroenterol 2002; 122:1388-98. [ Links ]
33. Lee DC, Durrani AA, Chapman J. A difference infrared spectroscopic study of gramicidin a, alamethicin and bacteriorhodopsin in perdeuterated dimyristoyl phosphatidylcholine. Biochim Biophys Acta 1984; 769:49-56. [ Links ]
34. Kirichenko AV, Masan KA, Straume M, Teater CD, Rich TA. Nuclear scintigraphic assessment of radiation-induced intestinal dysfunction. Radiol Res 2000; 153:164-72. [ Links ]
![]() Correspondence:
Correspondence:
Dr. Sankar Nath Sanyal.
Dept. of Biophysics.
Panjab University.
Chandigarh-160014, India.
E-mail: sn.sanyal@yahoo.com
Recibido: 21-III-2007.
Aceptado: 12-IV-2007.