My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.23 n.6 Madrid Nov./Dec. 2008
Effects of dietary supplementation with medicinal fungus in fasting glycemia levels of patients with colorectal cancer: a randomized, double-blind, placebo-controlled clinical study
Efectos de la suplementación dietética con hongos medicinales en la glicemia de ayuno de enfermos con cáncer colorrectal: ensayo clínico aleatorizado, doble-ciego, placebo-controlado
R. C. Fortes1, V. L. Recôva2, A. L. Melo2 and M. R. C. G. Novaes3
1Science and Education School Sena Aires-GO. University of Brasilia-DF. Brazil. 2Institute of Health Science (ESCS/FEPECS/SESDF). Brasilia-DF. Brazil. 3School of Medicine. Institute of Health Science (ESCS/FEPECS/SESDF). University of Brasilia-DF. Brazil.
Dirección para correspondencia
ABSTRACT
Objective: The objective of this study was to evaluate the effects of dietary supplementation with Agaricus sylvaticus medicinal fungus in fasting glycemia of post-surgery patients with colorectal cancer.
Scope: Proctology Ambulatory of Federal District Base Hospital-Brazil.
Subjects: Fifty-six colorectal cancer patients in postsurgery phase, stadiums phase I, II and II, which followed specific criteria of inclusion and exclusion.
Interventions: All patients were randomized in two groups: supplemented with Agaricus sylvaticus fungus (30 mg/kg/day) and placebo, and were followed up for six months. Subjects were divided later on according to BMI-Body Mass Index, sex and stage. Three fasting glycemia evaluations were carried out throughout the treatment.
Results: Subjects presented BMI medium = 24.65 kg/m2 (57.1% women and 42.9% men). The placebo group (average age 59.14 ± 12.95 years) had initial glycemia levels of 94.36 ± 15.34 mg/dL, after three months 98.12 ± 15.54 mg/dL (p = 0.03) and in the sixth month 98.52 ± 9.03 mg/dL (p = 0.01). The supplemented group (average age of 56.34 ± 15.53 years) had initial glycemia levels of 95.92 ± 11.64 mg/dL, after three months 94.88 ± 12.24 mg/dL (p = 0.65) and, in the sixth month, a significant reduction to 92.86 ± 6.82 mg/dL (p = 0.01).
Conclusion: The results suggest that the dietary supplementation with Agaricus sylvaticus medicinal fungus can significantly reduce fasting glycemia levels of colorectal cancer patients in post-surgery phase.
Key words: Medicinal fungus. Agaricus sylvaticus. Hypoglycemic substances. Colorectal cancer.
RESUMEN
Introducción: El objetivo de este estudio fue evaluar los efectos en la glicemia de ayuno de los enfermos con cáncer colorrectal, en fase post-operatoria, con suplementación dietética con hongos medicinales Agaricus sylvaticus.
Ámbito: Ambulatorio de Proctología del Hospital de Base do Distrito Federal-Brasil.
Sujetos: Cincuenta y seis enfermos con cáncer colorrectal en la fase post-operatoria, estadio I, II y III, siguiendo criterios de inclusión y exclusión.
Intervenciones: Todos los enfermos fueron aleatorizados en dos grupos: suplementado con hongos Agaricus sylvaticus (30 mg/kg/día) y placebo, por seis meses. Enfermos fueron divididos siguiendo el Índice de Masa Corporal (IMC), sexo y estadio. Tres evaluaciones de glicemia de ayuno en el tratamiento.
Resultados: Los enfermos presentaban IMC promedio de = 24,65 kg/m2 (mujeres 57,1% y hombres 42,9%). El grupo placebo (edad 59,14 ± 12,95 años) tenía glicemia inicial de 94,36 ± 15,34 mg/dL, después de tres meses 98,12 ± 15,54 mg/dL (p = 0,03) y en el sexto mes presentó aumento de 98,52 ± 9,03 mg/dL (p = 0,01). El grupo suplementado (edad de 56,34 ± 15,53 años) tenía glicemia inicial de 95,92 ± 11,64 mg/dL, después de tres meses 94,88 ± 12,24 mg/dL (p = 0,65) y en el sexto mes, reducción significativa de 92,86 ± 6,82 mg/dL (p = 0,01).
Conclusión: Resultados sugieren que la suplementación dietética con hongos Agaricus sylvaticus puede reducir la glicemia de ayuno de los enfermos con cáncer colorrectal en la fase post-operatoria, presentando efectos benéficos en el prognóstico de los enfermos.
Palabras clave: Hongos medicinales. Agaricus sylvaticus. Hipoglicemia. Cáncer colorrectal.
Introduction
Colorectal cancer is the second highest cause of death from cancer in western world.1 In the United States alone, it represents the third most common cause of cancer, reaching about 150,000 cases annually, resulting in 57,000 deaths.2 In Spain, it is the second cause of death from malignant neoplasia and the first one in populations that possess smoking habits.3 In Brazil it is the fifth most diagnosed neoplasia being the fourth cause of death.4
Death rates from colorectal cancer are higher in overweight individuals, (Body Mass Index, BMI ≥ 25 and < 30 kg/m2) or obesity (BMI ≥ 30 kg/m2) in both genders, if compared with normal weight individuals (BMI ≥ 18,5 and < 25,0 kg/m2).2,5 Other studies demonstrate close link between patients with colorectal cancer and high prevalence of overweight and obesity2,6,7 and deposit of visceral fat.7,8
Diverse factors, besides obesity, contribute to the high risk of colorectal cancer such as age, family history, 2,7,9,10 physical inactivity, alcohol consumption10-12and typical western diets.9,10,11,13
Inadequate dietary habits and lifestyle factors are associated with peripheral resistance to insulin and, consequently, to hyperinsulinemia, further to high levels of insulin-like growth factor (IGF-1).7,10,14 Hyperinsulinemia is related directly to the carcinogenesis process since it can stimulate colorectal tumor growth.11,15-19 Likewise, the insulin-like growth factor is responsible for the proliferation and apoptosis, being able to influence carcinogenesis significantly.7,14,17,18
Some researchers suggest that the medicinal fungus or its extracts, when used as dietary supplement, show hypoglycemic activity in experimental and clinical trials,20-25 since it contains substances that seem to act in the regulation mechanism of glucose metabolism.26
The objective of the present study was to assess the effects of a dietary supplementation with Agaricus sylvaticus fungus in fasting glycemia in post-surgery patients with colorectal cancer treated at the ambulatory of a pubic hospital in the Federal District, Brazil.
Methods
Study design
The study consists of a randomized, double-blind, placebo-controlled clinical trial, with random allocation of subjects. It was approved by the Research Ethics Committee of the Health Ministry-Federal District-Brazil. Terms of free consent was obtained from patients, whose participation was voluntary, after they acknowledged the procedures of the study. The work was developed in the ambulatory of proctology of the Base Hospital of the Federal District, a Public Hospital in Brazil, from november 2004 to july 2006.
The sample
The sample consisted of 56 patients (24 men and 32 women), with colorectal cancer, stadiums I (n = 12), II (n = 16) and III (n = 28), divided in two groups: placebo and supplemented with Agaricus sylvaticus fungus. Were included in the study patients with colorectal cancer in post-surgery phase, from three months to two years of surgical intervention, twenty years old or older; were excluded pregnant women, breast feeding infants, patients physically disable, patients in use of alternative therapy, patients with any other non transmissible chronic disease or undergoing metastasis process.
Agaricus sylvaticus extract
The Agaricus sylvaticus fungus, whose popular name is Sun Mushroom, was obtained from a producer licensed by the Brazilian Agropecuary Company-Embrapa, from the Tapiraí area, in the countryside of São Paulo State. The fungus extract was obtained by soaking dehydrated material in hot water for 30 minutes, liquefied, bolted and dried in a drying box. The chemical composition of the final solution was analyzed by the Japan Food Research Laboratories Center by HPLC method and the results showed the presence of carbohydrates (18.51 g/100 g), lipids (0.04 g/100 g), ergosterol (624 mg/100 g), proteins (4.99 g/100 g), amino-acids (arginine-1.14%; lysine-1.23%; histidine-0.51%, phenylalanine-0.92%, tyrosine-0.67%, leucine-1.43%, methionine-0.32%, valine-1.03%, alanine-1,28%, glicine-0.94%, proline-0.95%, glutamic acid -3.93%, serine-0.96%, threonine-0.96%, aspartic acid-1.81%, tryphtophan-0.32%, cysteine-0.25%) and micronutrients in trace quantities.
The dried extract was transformed into tablets, following pharmacotechnical procedures and the dosage supplemented for the group was equivalent to 30 mg/kg/day (six tablets per day -three in the morning and three in the afternoon between meals- divided into two daily intakes), considering the average weight of the studied population, during a period of six months. The placebo group received the same tablets with resembling ingredients and the same amount of calories, but without Agaricus sylvaticus extract.
Clinical evolution
The patients were monitored for six months. During the first tree months consultations were set up every 15 days for clinical evaluation and in the final tree months, every 30 days.
All patients remained with their usual diet, but during the treatment they received general orientation on how to keep a healthy diet. After six months of accompaniment, all patients received a personal diet and were sent to other health professionals when necessary.
The patients performed three fasting plasma glucose laboratorial tests: one before beginning the supplementation, another one after three months of treatment and the last one at the end of the treatment (after six months).
All patients were contacted by the researchers weekly by telephone for clarifications of doubts, checking he adequate use of the mushroom according to orientations and confirmation of the appointment, guaranteeing major adhesion to the treatment and control on the continuity of the study.
Were considered drop outs: patients who showed up at the first appointment only; the ones who did not come to consultations during the six-month period; the ones who underwent less than three examinations. Those who died before the end of the treatment were not included in the sample.
Laboratorial evaluation
The collection of the blood was realized following the criterion of 12 h fasting patients. The collected material was placed in vacuum tubes for serum obtention, having followed the protocols recommended by the Brazilian Society of Pathology for Collection of Venus Blood.27 The examinations were carried through at the Base Hospital Laboratory of Clinical Pathology of the Health Ministry-Federal District. The collected samples were centrifuged and analyzed in a 3000 TARGA device - Random Access Chemistry Analyzer, following laboratorial procedures. Analyses were determined by the enzymatic method in photocolorimeter pipes using Wiener kits. The reagents used contained glucose-oxidase, peroxidase, 4-aminofenazona and drain plugs phosphate.
Statistical analysis
The patients were divided in groups according to the BMI presented at the end of the treatment.28 A group of eutrophic patients and a group of overweight and obese patients were separated and analyzed later on by gender (women and men) and stadium (stadium I, II and III).
Values of the fasting plasma glucose test presented, were compared and analyzed, using the t-student and F statistical test, realized by the Microsoft, Excel version 2003 and SPSS (Statistical Package of the Social Sciences, SPSS Inc., Chicago, USA) for Windows version 14.0 programs with value of significance for p ≤ 0.05.
Results
After six months of monitoring at the ambulatory of proctology of the Base Hospital-Federal District-Brazil, 56 patients with colorectal cancer had concluded the study, being 32 women (57.1%) and 24 men (42.9%) divided in two groups, placebo and Agaricus sylvaticus.
In the placebo group (n = 28) the average age was 59.14 ± 12.95 years. In relation to gender, 57.1% (n = 16) there were 16 women, three of stadium I, seven of II and six of III, and 42.9% (n = 12) and 12 men, one stadium I, three of II and eight of III. The average body mass level of this group was of 23.99 ± 4.11 kg/m2, indicating the BMI within the eutrophic or normal range (BMI ≥ 18.5 e < 25.0 kg/m2) and overweight (BMI ≥ 25.0 e < 30.0 kg/m2).
Patients from the group that received Agaricus sylvaticus (n = 28) were aged an average of 56.34 ± 15.53 years. As the gender, 57.1% (n = 16) were women, six of the stadium I, two of II and eight of III, and 42.9% (n = 12) were men, two of stadium I, four of II and six of stadium III. The average BMI of the group was of 24.76 ± 4.10 kg/m2, indicating eutrophic and overweight.
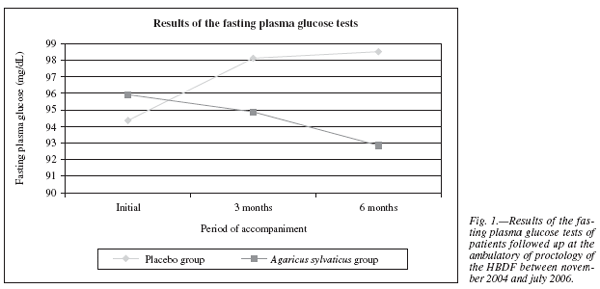
In relation to the fasting plasma glucose test, the results observed were: the placebo group initially had an average glucose concentration of 94.36 ± 15.34 mg/dL, after three months a significant increase of glucose to 98.12 ± 15.54 mg/dL (p = 0.03) occurred, remaining at normal levels (70-110 mg/dL); during the sixth month of supplementation it was observed again a significant increase in the plasma glucose concentration to 98.52 ± 9.03 mg/dL (p = 0.01), remaining within normal levels (fig. 1).
The group supplemented with Agaricus sylvaticus had initially an average glucose concentration of 95.92± 11.64 mg/dL, after three months it was observed a reduction to 94.88 ± 12.24 mg/dL, however not statistically significant (p = 0.65). In the sixth month of supplementation a more significant reduction of fasting plasma glucose levels occurred, from 94.88 ± 12.24 mg/dL to 92.86 ± 6.82 mg/dL (p = 0.01) (fig. 1).
The relation between plasma glucose and BMI in the placebo group, suggests that eutrophic patients (n = 14) presented better results of fasting glucose in the beginning of the treatment, 91.93 ± 18.50 mg/dL when compared to overweight patients (n = 11) and obese (n = 3), 97.15 ± 10.70 mg/dL and after 6 months, a statistically significant increase of plasma glucose was observed in eutrophic patients p = 0.02 (of 91.93 ± 18.50 mg/dL to 96.31 ± 9.16 mg/dL), and not significant in overweight and obese patients, p = 0.25 (of 97.15 ± 10.70 mg/dL to 100.92 ± 8.62 mg/dL).
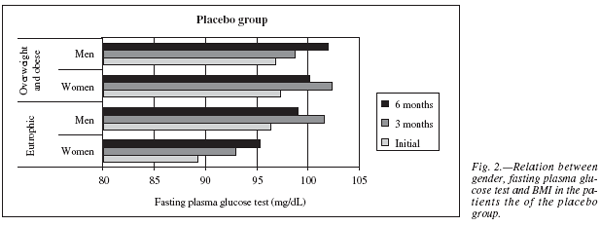
When the groups were separated by gender it could be observed that women in the eutrophic group (n = 8) had lower initial glucose levels, 89.25 ± 12.09 mg/dL, than the overweight and obese patients (n = 6), 97.38 ± 13.26 mg/dL. After six months, an increase of glucose levels were evidenced in both groups, this time statistically significant in the eutrophic group with p = 0.05 (of 89.25 ± 12.09 mg/dL to 95.29 ± 7.36 mg/dL), and not significant in the overweight and obese group, p = 0.62 (of 97.38 ± 13.26 mg/dL to 100.14 ± 9.77 mg/dL). Men of the eutrophic group (n = 6) and the overweight or obese group (n = 5) had practically the same levels of initial fasting plasma glucose concentration (of 96.33 ± 13.71 mg/dL to 96.8 ± 5.97 mg/dL, respectively). After 6 months, both groups presented increase in glucose, but it was higher in the overweight or obese group (of 96.80 ± 5.97 mg/dL to 102.00 ± 7.65 mg/dL, p = 0.22) than that of the eutrophic group (of 96.33 ± 13.71 mg/dL to 99.00 ± 11.55 mg/dL, p = 0.74), these results being not statistically relevant (fig. 2).
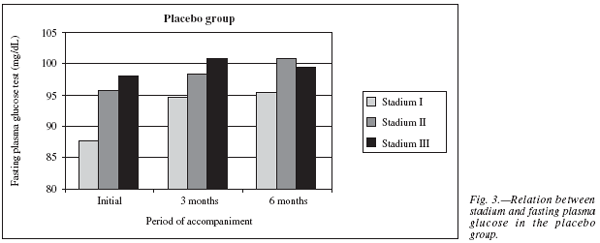
In relation to glucose and stadium in the placebo group, it was observed that the patients in stadium I (n = 4) had lower initial glucose levels than patients of stadium II (n = 10) and III (n = 14), 87.67 ± 16.80 mg/dL, 95.64 ± 6.76 mg/dL, 98.00 ± 16.35 mg/dL, respectively. In the sixth month of treatment all three groups presented an increase in glucose levels, being that patients of stadium I kept lower levels (95.33 ± 10.69 mg/dL, p = 0.16, against 100.73 ± 8.99 mg/dL, p = 0.09 of stadium II and 99.45 ± 8.80 mg/dL, p = 0.06 of stadium III) (fig. 3).
In the group treated with Agaricus sylvaticus, the relation between plasma glucose and BMI demonstrated that the eutrophic patients (n = 11) had fasting glucose results of 95.00 ± 12.30 mg/dL and the overweight patients (n = 14) obese (n = 3), of 97.67 ± 11.00 mg/dL in the beginning of the treatment. After 6 months of supplementation, reduction of plasma glucose was observed in the eutrophic patients (of 95.00 ± 12.30 mg/dL to 91.00 ± 8.70 mg/dL) and in the overweight and obese patients (of 97.67 ± 11.00 mg/dL to 96.93 ± 7.56 mg/dL), however, these are not statistically significant, with p = 0.34 and p = 0.09 respectively.
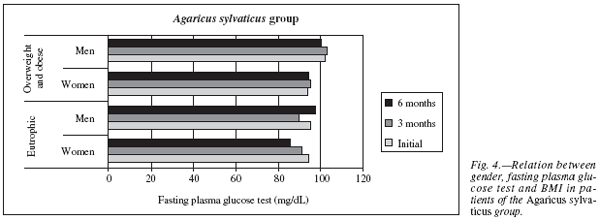
As we separated the supplemented group with Agaricus sylvaticus according to gender and BMI, it was observed that the eutrophic women (n = 8) had approximately the same levels of fasting plasma glucose as the patients from the overweight or obesity group (n = 6), 94.67 ± 13.59 mg/dL and 93.75 ± 10.19 mg/dL, respectively. At the end of the supplementation period, the eutrophic patients had a reduction of glucose (of 94.67± 13.59 mg/dL to 85.60 ± 5.94 mg/dL), however this was not statistically relevant, p = 0.34. In the overweight or obese group it was observed consistency of values of fasting plasma glucose (of 93.75 ± 10.19 mg/dL to 94.13 ± 6.4 mg/dL, p = 0.90). As for men, the eutrophic (n = 5) presented initial glucose of 95.50 ± 12.01 mg/dL, the overweight or obese (n = 8) of 102.14± 10.78 mg/dL. At thee end of the treatment, an increase of the glucose levels was observed in the eutrophic group (of 95.50 ± 12.01 mg/dL to 97.75 ± 6.70 mg/dL), however this was not statistically relevant p = 0.46, in the group with overweight or obese was observed a reduction of the glucose levels of 102.14 ± 10.78 mg/dL to 100.67 ± 5.57 mg/dL, however this was not statistically significant, p = 0.97 (fig. 4).
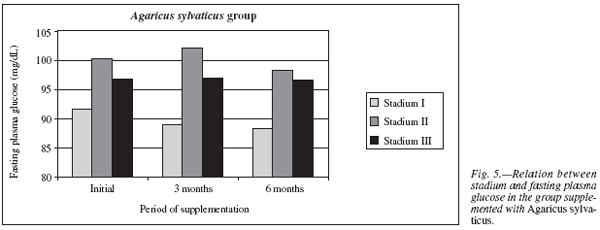
Comparing the levels of fasting plasma glucose and stadium in the Agaricus sylvaticus group, it was observed that the patients in stadium I (n = 8) had lower glucose levels (91.56 ± 8.19 mg/dL) than patients of stadium II (n = 6, 100.17 ± 11.48 mg/dL) and III (n = 14, 96.73 ± 14.14 mg/dL). After six months of supplementation, patients in all stadiums had glucose reduction. In stadium III of 96.73 ± 14.14 mg/dL to 95.6 ± 5.02 mg/dL, p = 0.05 reductions were statistically relevant, in patients of stadium I from 91.56 ± 8.19 mg/dL to 88.33 ± 5.39 mg/dL, p = 0.26 and stadium II from 100.17 ± 11.48 mg/dL to 98.17 ± 9.64 mg/dL, p = 0.66 reductions in final results were not statically relevant (fig. 5).
Discussion
In the present study, patients supplemented with Agaricus sylvaticus were characterized as adult (average age of 56.34 ± 15.53 years), eutrophic and overweight (average BMI of 24.76 ± 4.10 kg/m2).
The relation between cancer, obesity and hyperinsulinemia is fairly clear in the literature.29 Prevalence increase of abnormal carbohydrates metabolism, especially in relation to plasmatic glucose high levels and insulin, occurs with aging30-32 and overweight16,30 as observed in the placebo and Agaricus sylvaticus groups. Obesity is associated with hyperinsulinemia and growing risk of neoplasia such as breast, prostate and endometrium, but mainly colorectal.19,29 The hyperinsulinemia seems to be a determinant factor between this illness and several types of cancer.15,16,18,19,33 Measures can be taken to reduce plasmatic insulin and peripheral resistance to insulin as energy restriction, high fiber consumption and daily physical activities, all having preventive effect or delay on the growth of specific tumors.7,19
In obese individuals, the risk of carcinogenesis is statistically correlated with abdominal fat distribution. Abdominal obesity poses a higher risk of colorectal cancer than generalized obesity. In adults, weight gain culminates with abdominal obesity which in turn significantly increases the risk of colonic adenomas and colorectal carcinoma.7,10,29 However, in our study, the visceral adiposity was not inquired.
Some hypotheses such as, physical inactivity, high BMI, central adiposity, alcohol consumption and a typical Western diet might explain the accruement of colorectal cancer risk. The association of these factors with insulin resistance, hyperinsulinemia, and high levels of IGF may stimulate the growth of colorectal tumors.7,10,13,14,15,17
Experimental studies with animals indicate that insulin administration can promote carcinogenesis, since the multiplicity of focus of aberrant epithelial cells in the intestinal cryptas and the production of sialomucin in female rats, are augmented by the action of insulin, resulting in growth of intestinal epithelium.29
The action of insulin on its receptor can promote mitogenic stimulation for a long period of time. In normal physiological conditions it is observed the concentration reduction of the insulin receptor on the citoplasmatic membrane with an increase of plasmatic insulin levels. This fact does not occur in cancerous cells which remain with high receptors number independent of insulinemia levels. The insulin receptors of the neoplasic cells lose their capacity of under regulate the binding sites of insulin with consequent increase of tumoral sensitivity to the stimulatory effects of this hormone. This abnormal sub-regulation and the dissociation of the biological action of insulin can offer to the cancerous cells diverse metabolic advantages. Additional studies are needed with the purpose of investigating the role of insulin and insulin growth related to factors in the process of carcinogenesis.29
The metabolism of the oncological patients may present important alterations as consequence of the tumor's presence.34-36 It has been observed that cancer patients with alterations in the metabolism of carbohydrates is due mainly to the elevated glucose turnover, increase of gliconeogenesis12,37,38 and resistance to insulin, 36,37 facts that will most likely culminate with hyperglycemia.37
In this study, a significant reduction of fasting glycemia in colorectal cancer patients was observed after six months of dietary supplementation with Agaricus sylvaticus fungus. Inverse results were found in the placebo group, where a significant increase in glycemic levels was observed, suggesting that Agaricus sylvaticus fungus possesses substances capable of reducing glycemia.
The gravity of the nutritional state of patients with colorectal neoplasia depends on the tumor's type and evolutive stadium.3 To establish a correlation between fasting glycemia and stadium, it was attested that in both groups, patients from stadium I had lower glycemic levels than those of stadiums II and III. These findings are in agreement with the literature, once scientific evidences demonstrate that peripheral glucose absorption in cancer is found to be deficient even after the infusion of high doses of insulin and that all alterations were observed in more advanced stadiums of the disease which suggests that there is an increment of glucose turnover as the tumor expands.37 However, after six months of supplementation with Agaricus sylvaticus, all patients in the different stadiums of the illness presented reduction of glycemic levels. These results were not observed in patients of the placebo group, whose levels of glycemia had increased thou not significant, suggesting once more that the bio-actives substances present in Agaricus sylvaticus fungus are capable of reducing plasmatic glucose.
Experimental studies carried out with rats with type 1 diabetes have demonstrated that the administration of Agaricus campestris fungus has important hypoglycemic action9 through insulin secretion from the closing of the K+-ATP canals leading to membrane depolarization of pancreatic β-cells with consequent influx of Ca2+.39
The anti-hyperglycemic action of Agaricus bisporus was investigated and attested, demonstrating that this mushroom exerts its effect on insulin secretion and/or action.20
Other studies have demonstrated that soluble polysaccharides present in the Auricularia auricle-judae fungus are capable of promoting significant glycemia reduction, insulinemia and glycosuria, besides increasing intraperitoneal insulin tolerance and the content of hepatic glycogen in rats.40
A clinical trial carried out with 71 patients diagnosed with type 2 diabetes, treated with fractions of Ganoderma lucidum polysaccharides (1,800 mg, 3 times/day, for 12 months), showed significant reduction of average values of postprandial glycemia for 11,8 mmol L-1 in the supplemented group when compared with the placebo group.41
No studies have been found in the literature which have evaluated the effects of Agaricus sylvaticus fungus in carbohydrate metabolism; nonetheless, results have demonstrated that this fungus is capable of reducing fasting glycemic levels in patients with colorectal cancer. There has been enough evidence to attest the presence of bioactive substances such as lecithin, ergosterol, proteoglucans, glucans and arginine in Agaricaceae fungus. Scientific evidences show that the substance responsible for the pharmacological and nutritional attribute of mushrooms is the β-glucans.12,21,22,24,42
The ingestion of moderate amounts of fiber can improve the glycidic metabolism in eutrophic individuals and overweight,30 besides modifying the postprandial glycemia as well as insulin responses in individuals with or without diabetes.43,44 Studies demonstrate that a linear decrease in the glycemia levels occurred when the amount of ß-glucans was increased.45 The consumption of soluble fibers, particularly of β-glucans present in fungus, brings down insulin and postprandial glucose peak and its respective curves, promoting beneficial effects towards glucose tolerance.30,45
According to Bourdon et al.,44 fiber regulates the amount and local of digestive and absorptive of carbohydrates processes, consequently modifying alimentary and physiological responses to a determined food. When fibers containing viscous polysaccharides such as glucans are included in the meals, reduced glycidic absorption can be observed, modifying alimentary hormone responses thus propitiating slower carbohydrates absorption.
Even though the intrinsic mechanisms of alimentary fiber and the improvement of homeostasis of glucose haven't been totally detailed in the literature, it is recognized that this property is multifactorial, involving retardation of gastric emptying, absorption reduction of carbohydrates, production of short chain fatty acids, improvement of insulin sensitivity and alteration in hormonal secretion.7,46-48 These phenomena could be related to liquid retention caused by the presence of soluble fiber in the intestine; reduction of access to pancreatic enzyme in reaching the diet polysaccharides through the increase of quimo viscosity and reduction of glucose diffusion by enterocytes. In so doing, the fiber acts liberating the gastrointestinal inhibiting peptide, cholecystokinin and enteric glucagons hormones which together with parassympatic stimulation promote retardation of gastric emptying, increasing intestinal motility and the release of insulin by the pancreas.46
The results suggest that the dietary supplementation with Agaricus sylvaticus fungus is capable of significantly reduce fasting glycemia in colorectal cancer patients in postoperative phase, offering beneficial effects in the metabolism of carbohydrates these patients. Nevertheless, due to the lack of studies in literature, further randomized clinical trials such as this one, are still necessary to determine other clinical conditions for the adjuvant use of Agaricus sylvaticus to be beneficial.
References
1. Freeza EE, Warchtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut 2006; 55:285-91. [ Links ]
2. Heo M, Allison DB, Fontaine KR. Overweight, obesity, and colorectal cancer screening: disparity between men and women. BMC Public Health 2004; 4:53. [ Links ]
3. Farriol M, Pons M, Roca N, Martínez M, Delgado G. Quimioterapia pre-operatoria y nutrición parenteral total en la neoplasia de colon. Nutr Hosp 2006; 21(3):303-306. [ Links ]
4. Bin FC. Rastreamento para cáncer colorretal. Rev Assoc Med Bras 2002; 48(4):275-96. [ Links ]
5. Calle EE, Rodríguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003; 348(17): 1625-38. [ Links ]
6. Lagra F, Karastergiou K, Delithanasis I, Koutsika E, Katsikas I, Papadopoulou-Zekeridou P. Obesity and colorectal cancer. Tech Coloproctol 2004; 8:161S-163S. [ Links ]
7. Campos FG, Waitzberg AGL, Kiss DR, Waitzberg DL, Habr-Gama A. Diet and colorectal cancer: current evidence for etiology and prevention. Nutr Hosp 2005; 20(1):18-25. [ Links ]
8. Otake S, Takeda H, Suzuki Y, Fukui T, Watanabe S, Ishihama K et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin Cancer Res 2005; 11(10):3642-46. [ Links ]
9. Granados S, Quiles JL, Gil A, Ramírez-Tortosa MC. Lípidos de la dieta y cáncer. Nutr Hosp 2006; 21(Supl. 2):44-54. [ Links ]
10. Fortes RC, Recôva VL, Melo AL, Novaes MRCG. Hábitos dietéticos de pacientes com cáncer colorretal em fase pós-operatória. Rev Bras Cancerol 2007; 53(3):277-89. [ Links ]
11. Kim Y-I. Diet, lifestyle, and colorectal cancer: is hyperinsulinemia the missing link? Nutrition Reviews 1998; 56(9):275-9. [ Links ]
12. Fortes RC, Novaes MRCG. Efeitos da suplementação dietética com cogumelos Agaricales e outros fungos medicinais na terapia contra o câncer. Rev Bras Cancerol 2006; 52(4):363-71. [ Links ]
13. Ravasco P, Monteiro-Grillo I, Marqués Vidal P, Camilo ME. Nutritional risks and colorectal cancer in a portuguese population. Nutr Hosp 2005; 20(3):165-172. [ Links ]
14. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr 2001; 131:3109S-3120S. [ Links ]
15. Hsu IR, Kim SP, Kabir M, Bergman RN. Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr 2007; 86(3):867-71. [ Links ]
16. HagymÃisi K, Tulassay Z. Role of obesity in colorectal carcinogenesis. Orv Hetil 2007; 148(51):2411-6. [ Links ]
17. Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 2007; 86(3):836-42. [ Links ]
18. Pierartz CZ, Rozowsky, JN. Papel de la nutrición em la prevención del cáncer gastrointestinal. Rev Chil Nutr 2007; 33(1):8-13. [ Links ]
19. Cavalleira JBC, Saad MJA. Doenças associadas à resistência à insulina/hiperinsulinemia, não incluídas na síndrome metabólica. Arq Bras Endocrinol Metab 2006; 50(2):360-7. [ Links ]
20. Novaes MRCG, Fortes RC, Garcez LC. Cogumelos comestíveis da família Agaricaceae: aspectos nutricionais e atividade farmacológica no câncer. Rev Soc Bras Farm Hosp 2004; 5:15-20. [ Links ]
21. Hwang H-J, Kim S-W, Lim J-M, Joo J-H, Kim H-O, Kim H-M, et al. Hypoglicemic effect of crude exopolysaccharides produced by a medicinal mushroom Phellinus baumii in streptozotocin-induced diabetic rats. Life Sciences 2005; 76:3069-80. [ Links ]
22. Novaes MRCG, Fortes RC. Efeitos antitumorais de cogumelos comestíveis da família agaricaceae. Revista Nutrição Brasil 2005; 4(4):207-17. [ Links ]
23. Sullivan R, Smith JE, Rowan NJ. Medicinal mushrooms and cancer therapy. Spring 2006; 49(2):159-70. [ Links ]
24. Taveira VC, Novaes MRCG. Consumo de cogumelos na nutrição humana: uma revisão da literatura. Com Ciências Saúde 2007; 18(4):315-22. [ Links ]
25. Firenzuoli F, Gori L, Lombardo G. The medicinal mushroom Agaricus blazei Murrill: review of literature and pharmacotoxicological problems. Evid Based Complement Alternat Med 2008; 5(1):3-15. [ Links ]
26. Wasser SP, Weis AL. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: current perspectives. Int J Med Mushrooms 1999; 1:31-62. [ Links ]
27. Recomendações da Sociedade Brasileira de Patologia Clínica/Medicina Laboratorial para Coleta de Sangue Venoso. 1ª edição. São Paulo. 2005: 01-76. [ Links ]
28. World Health Organization. Physical status: the use and interpretation of anthropometry. WHO Technical Report Series 854. Geneva: WHO. 1995. [ Links ]
29. Halpern A, Mancini MC. Obesidade, hiperinsulinismo e câncer. In: Waitzberg DL. Dieta, nutrição e câncer. São Paulo: Atheneu; 2004: 734-38. [ Links ]
30. Behall AM, Schofield DJ, Hallfrisch JG, Liljeberg-Elmståhl HGM. Consumption of both resistant starch and beta-glucan improves postprandial plasma glucose and insulin in women. Diabetes Care 2006; 29:976-81. [ Links ]
31. Anisinov VN. Biology of aging and cancer. Cancer Control: Journal of the Moffitt Cancer Center 2007; 14(1):23-31. [ Links ]
32. Anisinov VN. Carcinogenesis and aging 20 years after: escaping horizon. Mech Ageing Dev. In press 2008. [ Links ]
33. Suba Z, UpjÃil M. Disorders of glucose metabolism and risk of oral cancer. Fogorv Sz 2007; 100(5):243-9. [ Links ]
34. Argilés JM, Busquets S, López-Soriano FJ, Figueras M. Fisiopatología de la caquexia neoplásica. Nutr Hosp 2006; 21(Supl. 3):4-9. [ Links ]
35. Planas M, Puiggrós C, Redecillas S. Contribución del suporte nutritional a combatir la caquexia cancerosa. Nutr Hosp 2006; 21(Supl. 3):27-36. [ Links ]
36. Cardona D. Tratamiento farmacológico de la anorexia-caquexia cancerosa. Nutr Hosp 2006; 21(Supl. 3):17-26. [ Links ]
37. Rivadeneira DE, Evoy D, Fahey III TJ, Liemberman MD, Daly JM. Nutritional support of the cancer patient. CA Cancer J Clin 1998; 48(2):69-80. [ Links ]
38. Faria FA, Campos CS, Fortes RC. Terapia nutricional enteral em pacientes oncológicos: uma revisão da literatura. Com Cien Saúde. 2008; 19(1):61-70. [ Links ]
39. Gray AM, Flatt PR. Insulin-releasing and insulin-like activity of Agaricus campestris (mushroom). Journal Endocrinology 1998; 157(2):259-66. [ Links ]
40. Yuan Z, He P, Cui J, Takeuchi H. Hypoglycemic effect of water-soluble polysaccharide from Auricularia auricula-judae Quel. on genetically diabetic KK-Ay mice. Biosci Biotechnol Biochem 1998; 62(10):1898-1903. [ Links ]
41. Lindequist U, Niedermeyer THJ, Jülich W-D. The pharmacological potential of mushrooms. eCAM 2005; 2(3):285-99. [ Links ]
42. Fortes RC, Taveira VC, Novaes MRCG. The immunomodulator role of beta-D-glucans as co-adjuvant for cancer therapy. Rev Bras Nutr Clin 2006; 21(2):163-8. [ Links ]
43. Braaten JT, Wood PJ, Scott FW, Riedel KD, Poste LM, Collins MW. Oat gum lowers glucose and insulin after an oral glucose load. Am J Clin Nutr 1991; 53(6):1425-30. [ Links ]
44. Bourdon I, Yokoyama W, Davis P, Hudson C, Backus R, Richter D et al. Postprandial lipid, glucose, insulin, and cholecystokinin responses in men fed barley pasta enriched with beta-glucan. Am J Clin Nutr 1999; 69:55-63. [ Links ]
45. Cavallero A, Empillit S, Brighenti F, Stanca AM. High (1-3,1-4)-beta-glucan barley fractions in bread making and their effects on human glycemic response. Journal of Cereal Science 2002; 36:59-66. [ Links ]
46. Catalani LA, Kang EMS, Dias MCG, Maculevicius J. Fibras alimentares. Rev Bras Nutr Clin 2003; 18:178-82. [ Links ]
47. Álvarez EE, Sánchez PG. La fibra dietética. Nutr Hosp 2006; 21(Supl. 2):61-72. [ Links ]
48. Weickert MO, Pfeiffer AF. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr 2008; 138(3): 439-42. [ Links ]
![]() Dirección para correspondencia:
Dirección para correspondencia:
Renata Costa Fortes.
QI 14. CJJ. CS 26. Guará 1.-DF.
71.015-100 DF. Brazil.
E-mail: renatacfortes@yahoo.com.br
Recibido: 5-XI-2007.
Aceptado: 9-VI-2008.