Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.23 no.6 Madrid nov./dic. 2008
Effects of soy milk as a dietary complement during the natural aging process
Efecto de la leche de soja como completemento dietario durante el proceso de envejecimiento natural
M. Fontenla1, A. Prchal2, A. M. Cena1, A. L. Albarracín2, S. Pintos1, S. Benvenuto1, M. L. Sosa1 and S. Fontenla de Petrino1
1Cátedra de Biología. Departamento Biomédico. Facultad de Medicina. Universidad Nacional de Tucumán. Argentina.
2Cátedra de Neurociencia. Departamento de Investigación. Facultad de Medicina. Universidad Nacional. Argentina.
ABSTRACT
Introduction: Oxidative stress is one mechanism that could contribute to the acceleration of aging and age-related diseases. On the other hand, because of their antioxidative qualities soybean derived foods could have beneficial effects on the aging process.
Objectives: The aim of our work was to study the effects of a diet supplemented with soy milk on certain biological features of aging in rats
Methods: Male Wistar rats of 3 to 18 months of age, were assigned to one of two diets: 1) Experimental Group, commercial rat formula and soy milk; 2) Control Group, commercial rat formula and water. Every three months both lipid profile and lipid peroxidation were determined and neuronal cells of hippocampus were counted in control and experimental rats.
Results: The soy milk diet significantly improved the plasmatic lipid profile, decreasing serum cholesterol (total as well as LDL) and serum triglycerides, HDL-cholesterol was significatively higher in experimental animals. The LDL/HDL ratio was thus significantly lowered. The soy diet also produced decreased values of lipid peroxidation in brain, liver and kidney. These effects were significant after 6 to 9 months. The experimental animals lost fewer hippocampal neurons than the controls. Finally at 18 months of age, a greater number of surviving animals in experimental group with respect to the control one was observed.
Conclusions: 1) soy intake could have beneficial effects as a complement of normal diet, but not as a replacement for animal proteins and 2) these effects are the result of a very long period (almost lifelong) of consumption of this diet.
Key words: Soymilk. Oxidative stress. Lipid profile. Neuronal bodies.
RESUMEN
Introducción: El estrés oxidativo es uno de los mecanismos que contribuye al envejecimiento y al desarrollo de enfermedades crónicas. Por otro lado, los alimentos a base de soja, por a sus cualidades antioxidantes podrían tener un efecto benéfico en este proceso.
Objetivos: El propósito de nuestro trabajo fue estudiar el efecto de la suplementación dietaria con leche de soja sobre algunos parámetros biológicos del envejecimiento natural, en ratas.
Material y métodos: Se trabajó con ratas Wistar macho, desde los 3 y hasta los 18 meses de edad. Los animales fueron asignadas a dos dietas: 1) grupo experimental: alimento balanceado para ratas y leche de soja, ad libitum; 2) grupo control: alimento balanceado y agua ad libitum. Cada tres meses, en ambos grupos se realizaron las siguientes determinaciones: a) perfil lipídico plasmático; b) lipoperoxidación (MDA) en homogenato de cerebro, hígado y riñón, y c) recuento del número de cuerpos neuronales en el hipocampo.
Resultados: La alimentación con leche de soja mejoró notablemente el perfil lipídico: se encontró una disminución significativa de colesterol total, LDL-colesterol, triglicéridos y el índice LDL/HDL. Asimismo, se observó, en hígado, cerebro y riñón, una reducción de la lipoperoxidación, que alcanzó valores significativos entre los 6 y 9 meses de alimentación. Los animales alimentados con leche de soja perdieron menos neuronas en el hipocampo en relación a los controles. Finalmente, se observó un mayor número de animales sobrevivientes, a los 18 meses de edad, en el grupo experimental con respecto al control
Conclusiones: La leche de soja podría ejercer efectos benéficos como complemento de la dieta normal, no en reemplazo de las proteínas animales. Estos efectos se observan después de un periodo prolongado de ingesta (casi toda la vida).
Palabras clave: Leche de soja. Estrés oxidativo. Perfil lipídico. Cuerpos neuronales.
Introduction
Aging is an inevitable biological process that affects all living organisms.1 Changes due to age have been attributed to factors such as development, genetic defects, environment, diseases, and inborn process.2 Diverse deleterious age related cellular and tissue changes accumulate and progressively impair functions and can eventually cause death. As a result, during adult life, all physiologic functions gradually decline: there is a diminished capacity for cellular protein synthesis, a decline in immune function, an increase in fat mass, a loss of muscle mass and strength, and a decrease in bone mineral density.3
Oxidative stress may contribute to aging acceleration and age-related degenerative diseases.4 Free radical reactions could be involved in production of the changes associated with the environment, disease and the intrinsic aging process. The rapidly growing number of scientists involved in studies on the role of free radical reactions in biological systems should assure future significant increases in the healthy, useful, life span of man.2
Several studies have found evidence of oxidative damage to many macromolecules: DNA, lipids, proteins, supporting the hypothesis that oxidative injury might act as one cause of the aging process.5,6
During aging there are multiple molecular, cellular, structural, and functional changes in the brain. Neural cells may respond to these changes adaptively, or they may succumb to neurodegenerativecascades that result in disorders such as Alzheimer's and Parkinson's diseases.7
Some of the longest living people on earth have made soy beans and soy products a part of their daily diet for centuries.8 The health benefits of soy products are well documented throughout the world and are currently being studied by the US Food and Drug Administration (FDA).9,10
Soybeans contain several nutritive attributes of high value. It has been found that the intake of soy foods is closely related with lowered occurrences of chronic diseases.11 There are many functional ingredients contained in soy foods such as soy protein, isoflavones, saponins, phytic acid, phytosterol, and phenolic acid. Compared to casein, soy protein has shown a greater antioxidative ability in preventing lipid oxidation.12 Sierens et al13 demonstrated that antioxidant species (isoflavonoids) may act to decrease oxidative damage to DNA, protein and lipids thus reducing the risk of coronary artery disease and cancer. The possible mechanisms of isoflavones, studied in animals and humans, include enhancement of bile acid excretion, reduced cholesterol metabolism, increased thyroid hormones, and reduced insulin to glucagon ratios.14 In addition, the use of soy protein supplement containing isoflavones improves cognitive function, bone mineral density and plasma lipids in healthy postmenopausal women when started at the age of 60 years or later.15 Finally, in a previous paper16 we found that the soy milk complemented diet, improved both, unspecificic and gut mucosa immune responses.
The aim of our work was to assess whether nutrition with soymilk, as a dietary complement has effects on the main biological features of aging. In order to do this, we examined the plasmatic lipid profile as indicative of vascular and coronary integrity; the lipid peroxidation by reactive oxygen species (ROS) due to oxidative stress and its relation with the loss of hippocampal neuronal bodies in the central nervous system. Also, the life span of experimental animals was determined.
Material and methods
Animals
Male Wistar rats (n = 80), three months old, supplied by the School of Medicine Central Animal Facilities (Universidad Nacional de Tucumán, Argentina) were used. The animals were housed in plastic cages, in a temperature-controlled room (21 ± 1 ºC) with a controlled 12-hour light-darkness cycle (light on at 7:00 AM). Liquids and food of the two diets used (see below) were available ad libitum. All experimental procedures were done in accordance with the relevant directives of the European Union (86/609/EEC) and the rules and recommendations of the FESSCAL (Federación de Sociedades Sudamericanas de la Ciencia de Animales de Laboratorio).
Experimental design
For 15 months (from 3 to 18 months of life) the animals were assigned to one of two diets: A) balanced food and water (control group); B) balanced food and soy milk (experimental group). Every 3 months, 4 rats from each group, control and experimental, were used for lipid profile and lipid peroxidation assay. Another 4 rats were used for central nervous system histological studies. A further set of 28 rats (14 controls and 14 in experimental groups) were used to determine the average life span (These rats were used for behavioural studies not reported here).
The commercial soy milk used in this study (AdeS, Unilever, Argentina) is elaborated from a non- transgenic variety of soy. Its components are: protein: 2,6 g%; carbohydrates: 4 g%; total fat: 1,5 g%; Essential fatty acids: a linoleic acid: w-6, 0.81g% ; linolenic acid, w-3, 0.13 g%; fiber: 0,5 g%; minerals: Ca, 48 mg%; Fe, 0,84 mg%; P, 48 mg%; Mg, 18 mg%; Na, 80 mg%; K, 150 mg%; vitamins: A, 40 microg%; B6 , 0,2 mg%; B9, 20 microg%; B12, 0,1 microg%; C, 15 mg/%; D, 0,3 microg%; E, 0,6 mg% Isoflavones, 6,4% (daidzein: 2,4 mg% and genistein: 4 mg%).
All animals received a commercial laboratory food (Cargill, Buenos Aires, Argentina). This commercial diet contains 24,6% of protein from animal and vegetal origin.
Lipid profile and lipid peroxidation assay
Four rats from each group were anesthetized with synergic association of Ketamine (Ketamine 50, HOLLIDAY-SCOTT S.A.), doses of 200 mg/kg and 2% Xylacine (Xilacine 2%® Alfasan, Woerden Holland) doses 7 mg/kg. Half of the Ketamine dose and Xilacine dose was injected intraperitoneally while the remaining Ketamine dose was intramuscularly injected. Peripheral blood samples were obtained from the jugular vein for biochemical assay and then the brain, the kidney and the liver were quickly removed in order to determine lipid perioxidation.
The serum was separated from peripheral blood samples within one hour from extraction. Serum was assayed for cholesterol (Colestat, Wiener Lab., Rosario, Argentina) and triglycerides (TG) (TG Color, Wiener Lab., Rosario, Argentina) by the enzymatic method. The high density lipoprotein (HDL-C) (HDL-Colesterol, Wiener Lab., Rosario, Argentina) and low density lipoprotein (LDL-C) (LDL-Colesterol, Wiener Lab., Rosario, Argentina) were separated from the serum selectively precipitating them by adding the low and the high molecular weight polymers, respectively. Also, the LDL-C: HDL-C ratio was calculated.
Lipid peroxidation was assessed by the measurement of malondialdehyde (MDA) levels on the basis that the MDA reacts with 2-thiobarbituric acid (TBA) according to Ohkawa et al.17 Thiobarbituric acid-reactive substances (TBARS) were estimated in the brain, liver and kidney.
Briefly, the removed tissues were weighted and washed with cold 0, 9% saline. Then they were cut into small pieces with scissors. Approximately 1 g of each sample was homogenized in 3,5 ml ice-cold 0,1% trichloroacetic acid (TCA) using a Polytron PT 3000 homogenizer, followed by centrifugation at 10,000 g for 10 min. The supernatants were used for the TBARS measures. The top layers were mixed with TCA/TBA solution, to be further incubated at 90 °C for 20 min. Then, the mixtures were cooled in an ice bath. The absorbance of MDA/TBA complex was determined at 530 nm by conventional spectrophotometry. The concentration of TBARS was determined using the extinction coefficient of 1.56 x 105M-1 cm-1 and it was expressed in nmoles/ml.
Counting of hippocampus neuronal bodies
Neuronal loss is a conspicuous feature of the aging process in the central nervous system. It is accepted that this lack of cell is responsible for the cognitive impairment of old age. Furthermore, the hippocampus has been repeatly postulated as the place in thenervous system where memory fixation occurs.
Cornu ammonis 2 and 3 (CA2 and CA3) layers have been demonstrated as necessary structures for several learning tasks in rats. That is why we evaluated the hippocampal neuronal loss in this work.
Under general anesthesia (Ketamine and Xilacine, same doses as previous groups) four animals from each group were perfused via the left cardiac ventricle, with 10% neutral formalin in 0,9% NaCl. The brains were extracted and postfixed in 4% paraformaldehyde in PBS (pH 7.4). Then, the samples were dehydrated and embedded in paraffin at 56 ºC. The hippocampus was visually identified and cut in 10mm serial sections with a microtome. Sections were stained with Toluidine blue. Neuronal bodies in the CA3 and CA2 hippocampus fields were counted in 100 fields (magnification 40x). The results were expressed as the mean ± SD of stained neurons per field (40x). Glia was excluded from the counts based on size and cytological characteristics. Ten sections of each brain were used.
Statistical analysis
All statistical calculations were carried out with Sigma Stat software package (Jandel Scientific, San Raphael, California, USA). Differences between diet groups were analyzed by one way analysis of variance (ANOVA), and Dunnet's test was used as a post-hoc test. Differences were considered significant if p < 0.05.
Results
The mean values of lipid profiles of both groups studied are shown in table I. Serum cholesterol, TG and VLDL concentrations were significantly (P < 0,05) lowered. Cholesterol and triglycerids decreased 24% at the 6th month of the diet and maintained these lowered values until the 18th month. However the levels of HDL-C showed a 13% increment after the ninth month of the soy diet compared with the control group. The levels of LDL-C were considerably decreased (p < 0.05), which is the reason why the ratio of LDL-C to HDL-C of the experimental group was lower than the control group (P < 0.05).
The production of thiobarbituric acid-reactive substances (TBARS) in brain, liver and kidney are shown in figure 1 a, b and c.
Both brain and liver homogenates of the experimental group presented TBARS values that were significantly lower (p < 0.05) than the control group. These levels decreased approximately 55% in brain and liver of rats fed with soy milk compared to the control group (fig. 2 a, b). In the kidney homogenates, TBARS levels displayed significant differences (p < 0.05) in relation to controls, after 12 months of feeding with soy milk (fig. 2).
Figure 2 shows the relation of the number of hippocampal neurons with age and diet. The experimental animals lost fewer neurons than the controls (p < 0.05), in hippocampus layers CA2 and CA3 during the experimental period.
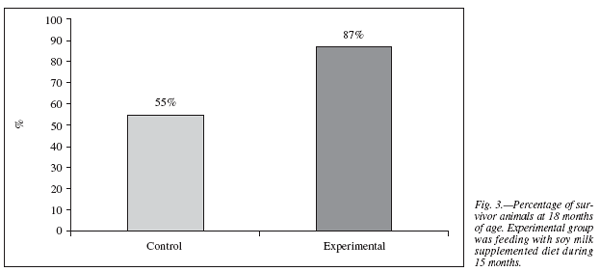
The life span average during 18 months is expressed as the percentage of surviving animals on each diet. A greater number of survivors among the animals fed with soymilk was registered with respect to the group that did not given soy milk . These results are presented in figure 3.
Discussion
Dietary components with antioxidant activity have received particular attention because of their potential role in modulating oxidative stress associated with aging and chronic deseases.18 In this context, taking into account the high nutritive value of soy foods and their antioxidant actions,19,20 we studied the effect of soy milk, as a complement to standard feeding, during the natural aging process in Wistar rats, from 3 to 18 months old. We used a commercial soymilk because it is a food that is within reach of practically all consumers.
Our results showed a beneficial effect of the diet complemented with soy milk on the plasma lipid profile: total cholesterol, LDL-cholesterol and triglyceride levels were significant lowered in the experimental group while the HDL-cholesterol was increased (table I). Of particular interest in this context is the reduced LDL-C:HDL-C ratio obtained, a strong predictor of cardiac events. Abnormal lipid levels contribute significantly to the risk of coronary heart disease, major cardiovascular disease and serious health problems. Our results suggest that the daily consumption of soy milk can significantly decrease serum cholesterol, LDL cholesterol and triglyceride concentrations, improving the LDL-C:HDL-C ratio.
These results could explain why soy protein consumption has been shown to significantly decrease serum concentrations of total and LDL cholesterol and triacylglycerols.21 Many components associated with soy protein, eg isoflavones,22 saponins23 and -conglycinin (7Sglobulin) protein fractions,24 were reported to have a lipid lowering effect. Hermansen et al25 in a review of more than 50 recent trials that included soy products consumption found that, besides a reduction of the total cholesterol, the LDL-C and the triglycerides, the relation LDL:HDL, was reduced by up to 27% with an average of 20%. It is probable that this relation itsef is more significant than the exclusive decrease of the LDL-C from the point of view of the benefit obtained for cardiovascular health. Similarly, Liao et al26reported that soy-based low-calorie diets significantly decreased total serum cholesterol and low-density lipoprotein cholesterol concentrations and had a greater effect on reducing the body fat percentage than traditional low-calorie diets.
Furthermore, Anderson et al27 informed that the consumption of soy protein rather than animal protein significantly decreased serum concentrations of total cholesterol, LDL cholesterol, and triglycerides but it was associated with a nonsignificant 2.4 percent increase in serum concentrations of high-density lipoprotein (HDL) cholesterol.
The question of the relation between the dietary complementation with soy milk and the oxidative stress during aging was assessed by the determination of the levels of lipid peroxidation in different tissues, expressed as thiobarbituric acid reactive substances (TBARS; MDA). Lipid peroxidation is a complex phenomenon that involves the generation of many products.28 Among them, MDA is one of the most significant end products of lipid peroxidation and its content, both in plasma and tissues, is generally accepted as an index of lipid peroxidation rate.29
Our results showed that TBARS levels decreased notably after the 6th month of feeding in both brain and liver (9 month old). The same results appeared in the kidney homogenates after the 9th month (fig. 1).
Similar results were reported by Soulsby et al.30 They determined that the MDA levels in brain tissues were decreased in the rats fed the soy diet and were increased in the rats that were tail-suspended to simulate weightlessness, when compared to those of rats on a regular diet. These observations imply that the soyprotein diet has a protective antioxidant effect during both the basaland the stressful condition.
Likewise, the liver and kidney TBARS concentrations were correlated with improvement of the plasmatic lipid profile. Also, the diminished kidney TBARS levels could explain, in part, the studies that have shown that soy protein diets limit or reduce proteinuria and kidney lesions associated with progressive kidney failure31,32.
Furthermore, the animals, whose diet was complemented with soy milk, lost less neuronal bodies than the controls, in layers CA2 and CA3 of hippocampus (fig. 2). We considered that the antioxidant effect of the soy milk supplement diminished the brain cellular injury, demonstrated by the decrease in lipoperoxidation. Perhaps, the diet supplements were able to significantly improve free radical scavenger systems. Consistentwith this role, recent studies have shown that Docosahexaenoic acid [22: 6 (n-3)] (DHA) has important free radical scavenging properties and protects against peroxidative damage of lipids and proteins in developing and adult brains,with attenuation of neuron loss and cognitive and locomotor deficits in animal models of ischemia-reperfusion brain injury33,34 Mammals35 obtain DHA either as DHA itself or the precursor a-linolenic acid [ALA, 18: 3 (n-3)], a fatty acid that the soymilk administered contains (see soy milk composition).
In addition to evidence36 that DHA may influence brain developmentthrough effects on gene expression, monoaminergic neurotransmission, or protection against apoptotic cell death, growth of neurite processes from the cell body is a critical step in neuronaldevelopment and involves a large increase in cell membrane surfacearea. The eventsin which DHA fulfils its essential roles, including protection from oxidative stress, are relevant throughout the lifespan and in maximizing cognitive potential in development and minimizing its loss with age.37 These effects would explain the increase in life span obtained in our experiments (fig. 3).
All together, our findings could explain - at least in part- both the action of soy proteins and isoflavones and the coayuvant action of the essential fatty acids, vitamins and minerals of the soy milk. It is important to note that the effects we reported here are the results of prolonged soy milk consumption as a dietary supplement. In others words, the beneficial effects appears only after a period of 3 to 9 months of chronic soy intake. Nine months is a period equivalent to 2/3 of the rat adult life (in the very protected conditions of an animal care facility).
Finally, the possibility that the effects we found in rats might also occur in the human being needs more extensive and controlled studies than the ones currently available. Nevertheless, our results could support several epidemiological works stressing the hypothesis that soy based or soy supplemented nutrition could have beneficial effects on health.
Acknowledgements
This work was financially supported by a grant from Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT) 26/I307-2005-2008, Argentina National University of Tucumán, Argentina.
The authors thank UNILEVER BESTFOODS (Argentina) for supplying the AdeS soy milk used in this work.
We also gratefully thank Patricia Black-Decima, PhD for helpful discussions during the preparation of the manuscript.
References
1. Yu BP. Aging and oxidative stress: modulation by dietary restriction. Free Radic Biol Med 1996; 21(5):651-668. [ Links ]
2. Harman D. The free radical theory of aging. Antioxid Redox Signal 2003; 5(5):557-61. [ Links ]
3. Lamberts S. Endocrinology Basic and Clinical Principles, Melmed, Shlomo HUMANA PRESS Second Edition. pp. 419. June 2005. [ Links ]
4. Liu J, Mori A. Stress, aging, and brain oxidative damage. Neurochem Res 1999; 24(11):1479-97. [ Links ]
5. Morillas J, García-Talavera N, Martín-Pozuelo G, Reina AB, Zafrilla P. Detección del riesgo de desnutrición en ancianos no institucionalizados. Nutr Hosp 2006; 21(6):650-656. [ Links ]
6. Fukagawa, Naomi K. Aging: Is Oxidative Stress a Marker or Is It Causal? Proc Soc Exp Biol Med 1999; 222:293-298. [ Links ]
7. Mattson MP, Chan SL, Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev 2002; 82(3):637-672. [ Links ]
8. Tilson WB. Proteins in a world of change. J Am Oil Chemists' Society 2007; 51(1):51A-52A. [ Links ]
9. AHS. Systems© 2007. http://www.soyenjoy.com/benefits.cfm [ Links ]
10. Farriol M, Jorda ME, Delgado G. Tendencias pasadas y actuales en la complementación con soja: un estudio bibliográfico. Nutr Hosp 2006; 21(4):448-451. [ Links ]
11. Adlercreutz CH, Goldin BR, Gorbach SL, Hockerstedt KA, Watanabe S, Hamalainen EK, Markkanen MH, Makela TH, Wahala KT, Adlercreutz T. Soybean phytoestrogen intake and cancer risk. J Nutr 1995; 125:757S-770S. [ Links ]
12. Ching YL, Zheng YT, I-Chi C, Shyh HL. Effects of fermented soy milk on the liver lipids under oxidative stress. World J Gastroenterol 2005; 11(46):7355-7358. [ Links ]
13. Sierens J, Hartley JA, Campbell MJ, Leathem AJ, Woodside. Effect of phytoestrogen and antioxidant supplementation on oxidative DNA damage assessed using the comet assay. Mutat Res 2001; 485(2):169-176. [ Links ]
14. Mokhtar IY, Kamel IK, Esmail A, Baghdadi H. Antioxidant activities and lipid lowering effects of isoflavone in male rabbits. Food and Chem Toxicol 2004; 42:1497-1503. [ Links ]
15. Kreijkamp-Kaspers S, Kok L, Grobbee D, F. de Haan E, Aleman A, Lampe J, Van der Schouw I. Effect of Soy Protein Containing Isoflavones on Cognitive Function, Bone Mineral Density, and Plasma Lipids in Postmenopausal Women. JAMA 2004; 292:65-74. [ Links ]
16. Fontenla de Petrino S, Prchal A, Fontenla M, Cena AM, Gómez J, Pintos S, Peral MC. Recovery from experimental malnutrition with soymilk: immunological and genetic aspects. Nutr Hosp 2007; 22(2):244-251. [ Links ]
17. Ohkawa H, Ohihi N, Yagi K. Assay for lipids peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1978; 95:351-358. [ Links ]
18. Meydani M. Nutrition interventions in aging and age-associated disease. Ann N Y Acad Sci 2001; 928:226-235. [ Links ]
19. Messina MJ. Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr 1999; Supl. 3: 439-450. [ Links ]
20. Wiseman H, O'Reilly JD, Adlercreutz H et al. Isoflavone phytoestrogens consumed in soy decrease F2-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am J Clin Nutr 2000; 72:395-400. [ Links ]
21. Scheiber MD, Liu JH, Subbiah MT, Rebar RW, Setchell KD. Dietary inclusion of whole soy foods results in significant reductions in clinical risk factors for osteoporosis and cardiovascular disease in normal postmenopausal women. Menopause 2001; 8:384-392. [ Links ]
22. Jenkins DJ, Kendall CW, Jackson CJ et al. Effects of high- and lowisoflavone soy foods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am J Clin Nutr 2002; 76:365-72. [ Links ]
23. Oakenfull DG, Sidhu GS. Could saponins be a useful treatment for hypercholesterolemia? Eur J Clin Nutr 1990; 44:79-88. [ Links ]
24. Adams MR, Golden DL, Franke AA, Potter SM, Smith HS, Anthony MS. Dietary soy beta-conglycinin (7S globulin) inhibits atherosclerosis in mice. J Nutr 2004; 134:511-516. [ Links ]
25. Hermansen K, Dinesen B, Hoie LH, Morgenstern E, Gruenwald J. Effects of soy and other natural products on LDL:HDL ratio and other lipid parameters: a literature review. Adv Ther 2003; 20(1):50-78. [ Links ]
26. Liao FH, Shieh MJ, Yang SC, Lin SH, Chien YW. Effectiveness of a soy-based compared with a traditional low-calorie diet on weight loss and lipid levels in overweight adults. Nutrition 2007; 23(7-8):551-556. [ Links ]
27. Anderson JW, Blake JE, Turner J, Smith B. Effects of soy protein on renal function and proteinuria in patients with Type 2 diabetes. Am J Clin Nutr 1998; 68(Supl.):1347S-53S. [ Links ]
28. Kagan VE. Lipid Peroxidation in Biomembranes. Boca Raton, FL: CRC Press, 1988. [ Links ]
29. Esterbauer H et al. Chemistry and Biochemistry of 4-Hydroxynonenal, Malondialdehyde and Related Aldehydes. Free Rad Biol Med 1991; 11:81-128. [ Links ]
30. Soulsby ME, Phillips B, Chowdhury P. Effects of Soy-Protein Diet on Elevated Brain Lipid Peroxide Levels Induced by Simulated Weightlessness. Ann Clin Lab Sci 2004; 34:103-106. [ Links ]
31. Velasquez MT, Bhathena SJ. Dietary phytoestrogens: a possible role in renal disease protection. Am J Kidney Dis 2001; 37(5):1056-1068. [ Links ]
32. Teixeira SR, Tappenden KA, Carson L et al. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J Nutr 2004; 134:1874-1880. [ Links ]
33. Cao D-H, Xu J-F, Xue R-H, Zheng W-F, Liu Z-L. Protective effect of chronic ethyl docosaehxaenoate administration on brain injury in ischemic gerbils. Pharmacol Biochem Behav 2004; 79:651-659. [ Links ]
34. Green P, Glozman S, Weiner L, Yavin E. Enhanced free radical scavenging and decreased lipid peroxidation in the rat fetal brain after treatment with ethyl docosahexaenoate. Biochem Biophys Acta 2001; 1532:203-212. [ Links ]
35. Innis SM. Perinatal biochemistry and physiology of long chain polyunsaturated fatty acids. J Pediatr 2003; 143:S1-8. [ Links ]
36. Sheila M. Innis Dietary (n-3) Fatty Acids and Brain Development. J Nutr 2007; 137:855-859. [ Links ]
37. Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci 2006; 29:263-271. [ Links ]
![]() Dirección para correspondencia:
Dirección para correspondencia:
Silvia Fontenla de Petrino.
José Colombres 255.
(4000) S. M. de Tucumán. Argentina.
E-mail: fontenla@arnet.com.ar; cbiologia@fm.unt.edu.ar
Recibido: 27-XII-2007.
Aceptado: 15-IV-2008.