Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.24 no.2 Madrid mar./abr. 2009
N-3 fatty acids in glucose metabolism and insulin sensitivity
Ácidos grasos n-3 en el metabolismo de la glucosa y la sensibilidad a la insulina
L. Martín de Santa Olalla1, F. J. Sánchez Muniz 2, and M. P. Vaquero1
1Department of Metabolism and Nutrition. Instituto del Frío. Instituto de Ciencia y Tecnología de Alimentos y Nutrición (ICTAN). Spanish Nacional Research Council (CSIC). Madrid. Spain.
2Department of Nutrition. Faculty of Pharmacy. Complutense University. Madrid. Spain.
This work was supported by the Spanish Ministry of Science and Innovation (projects AGL 2005-07204-C02-01/ALI and AGL2006-09519-ALI). LMSO was financed by I3P program financed by the European Social Funds.
ABSTRACT
Polyunsaturated fatty acids (PUFA) of the n-3 series are essential for normal growth and development. The health effects of these fatty acids include reduction of cardiovascular risk due to antiarrhythmic, antiinflammatory, anti-thrombotic and lipid lowering actions. An increase in unsaturation of the muscle membrane fatty acids is associated with improved insulin sensitivity. Higher proportion of n-3 fatty acids may have beneficial roles, such as antiobesity effects and protection against the metabolic syndrome and type 2 diabetes mellitus through a number of metabolic effects. However, controversy exists on the different effects of n-6 and n-3 polyunsaturated fatty acids as well as on the interacting effect of dietary saturated and monounsaturated fat. In addition, some adverse effects have been described concerning the use of fish oil supplements containing high doses of n-3 fatty acids. Several studies show Eskimos diabetes risk, while results of nutritional interventions on the influence of consuming diets rich in oily fish or other food rich in n-3 fatty acids is very limited. This article reviews the possible mechanisms through which n-3 PUFA are involved in glucose level control and insulin sensitivity. Intervention and epidemiological studies together with recent findings on the nutrigenomic field related with this subject are also briefly reviewed.
Key words: N-3 fatty acids. Fish oil. Type 2 diabetes mellitus. Insulin resistance. Nutrigenomics.
RESUMEN
Los ácidos grasos poliinsaturados (AGP) de la serie n-3 son esenciales para el crecimiento normal y el desarrollo. Los efectos de estos ácidos grasos en la salud incluyen reducción del riesgo cardiovascular debido a acciones antiarrítmicas, antiinflamatorias, antitrombóticas e hipolipemiantes. Un aumento en la insaturación de los ácidos grasos de la membrana muscular se asocia con mejora en la sensibilidad a la insulina. Una mayor proporción de ácidos grasos n-3 puede desempeñar papeles beneficios, tales como efectos antiobesidad y protección frente al síndrome metabólico y la diabetes mellitus tipo 2. Sin embargo, existe controversia sobre los diferentes efectos de los ácidos grasos n-6 y n-3, así como con la interacción entre la grasa saturada y monoinsaturada. Además, se han descrito algunos efectos adversos en relación al uso de suplementos de aceite de pescado con altas dosis de ácidos grasos n-3. Algunos estudios muestran el riesgo de diabetes en esquimales, mientras que los resultados de intervenciones nutricionales sobre la influencia del consumo de pescado graso u otros alimentos ricos en ácidos grasos n-3 son muy limitados. Este artículo revisa los posibles mecanismos a través de los cuales los AGP n-3 intervienen en el control de la glucosa y la sensibilidad a la insulina. Se revisan los estudios epidemiológicos y de intervención junto con los hallazgos más recientes en el campo de la nutrigenómica relacionados con la sensibilidad y resistencia a la insulina.
Palabras clave: Ácidos grasos n-3. Aceite de pescado. Diabetes mellitus tipo 2. Resistencia a la insulina. Nutrigenómica.
Background
The diet on which humans have evolved contains a ratio of n-6 to n-3 of ~1 whereas nowadays this has been discontinued, which leads to an upset in the functioning of some of our systems.1 A n-6/n-3 ratio of 4/1 to 6/1 is considered more suitable, although some authors propose a ratio of 1/1.2 The adoption of westernized lifestyle, characterized by low physical activity and high fat and salt intake, predisposes humans to the development of the metabolic syndrome (MetS), a major public health problem.3,4 This syndrome includes a series of metabolic disturbances combining insulin resistance, cardiovascular disease and obesity in the same individual.5,6 Dietary fat contains a variety of saturated, monounsaturated and polyunsaturated triacylglycerols,2,7 and the role of n-3 fatty acids on glucose levels control is here revised.
Therefore, the aim of the present work is to update existing knowledge on the role of n-3 fatty acids on the glucose metabolism and insulin resistance, and its relationship to the development of some chronic diseases, with special focus on the MetS and type 2 diabetes mellitus (T2DM).
Fatty acids: sources, elongation and desaturation
Fatty acids are the characteristic components of some lipids, composed of a hydrocarbon linear long chain mostly with an even number of carbon atoms and with a carboxylic edge. They are classified into saturated fatty acids (SFA), with simple binding between the carbons, such as palmitic (C16:0) and stearic (C18:0) acids; solids at room temperature; and unsaturated fatty acids, with double bonds between the carbons, which consist of monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA).2 There are three main families of PUFA, namely, n-9, n-6 and n-3 (table I).8,9 The distinction between n-3, n-6, and n-9 fatty acids is based on the location of the first double bond, counting from the methyl end of the fatty acid molecule. MUFA are represented by oleic acid (C18:1, n-9). Its double bond is between the 9th and 10th carbon atoms, thus it is an n-9 fatty acid. Oils rich in MUFA and PUFA are liquid at room temperature. The fatty acids of the n-3 and n-6 series are also known as essential fatty acids because humans, like all mammals, cannot synthesize them and must obtain them from their diet.8 Linoleic acid (LA, C18:2, n-6) is the mother fatty acid of the n-6 series while α-linolenic (ALA, C18:3, n-3) of n-3 series. Docosahexaenoic (DHA, C22:6, n-3), eicosapentaenoic (EPA, C20:5, n-3), and arachidonic (AA, C20:4, n-6) acids are PUFA playing very important physiological roles.
The n-3 and n-6 families of PUFA are important components of practically all cell membranes.1 Whereas cellular proteins are genetically determined, the PUFA composition of cell membranes is to a greatextent dependent on dietary intake.8
Although LA and ALA are the really essential fatty acids in an adult's diet, long chain PUFA (LCPUFA), DHA and AA, are also essential for the foetus' complete development as foetal desaturases and elongases are partially inefficient.3 Therefore, foetus essential fatty acids and LCPUFA have to come from the mother,who obtains them mostly from her diet.3
Table II shows the most accepted desaturation-elongation pathway for n-3 and n-6 PUFA. The existence of Δ4 desaturase has been discussed and the transformation of C22:4 n-6 or C22:5 n-3, into C22:5 n-6 and C22:6 n-3 respectively, has been attributed to a combination of the elongase-descarboxilase system.2,3
Dietary sources of ALA are mainly green leafy vegetables, linseed (also called flaxseed), soybean, canola, rapeseed oils, walnuts and Brazil nuts. N-3 LCPUFA, especially EPA and DHA, are found in oily fish, either from marine or farm origin, such as salmon, sardines, mackerel and tuna. N-6 PUFA occurs mostly in the diet as LA and can be found in sunflower oil, corn, rapeseed, safflower, oils, and many nuts, grains and seeds (table I).1,8,10
Insulin resistance and the metabolic syndrome
Insulin resistance is usually defined on a metabolic level as inefficient insulin function in skeletal muscle, liver and adipocytes. This hampers the normal role of insulin whereby it causes increased muscle cellular glucose uptake, glycogen synthesis, and cessation of hepatic glucose production.11,12 Table III summarizes the methods and indexes used to assess insulin resistance.
Insulin resistance is a growing worldwide phenomenon, which has progressively developed over years, and finally, if unchecked, predisposes to cardiovascular disease and T2DM.13,14 In current present society this is the consequence in lifestyle changes during the infant and adolescent stages: a decrease in physical activity while increasing body weight and nutrition changes (quick spread and success of fast food instead of the traditional and home-made cooking). Those changes have led to a loss of blood glucose control that might result from failure of the beta cells to secrete insulin, resistance of the tissues to its action, or a combination of both. This is because under a high-fat and high-sucrose diet insulin secretion increases to accommodate the need to store glucose and excess fatty acids.14-20 The condition of insulin resistance is tightly coupled with obesity and cardiovascular pathology; these conditions are collectively called "the MetS" or decades ago "syndrome X". 11,20,21 Recently it is also called Cardiometabolic Symdrome.
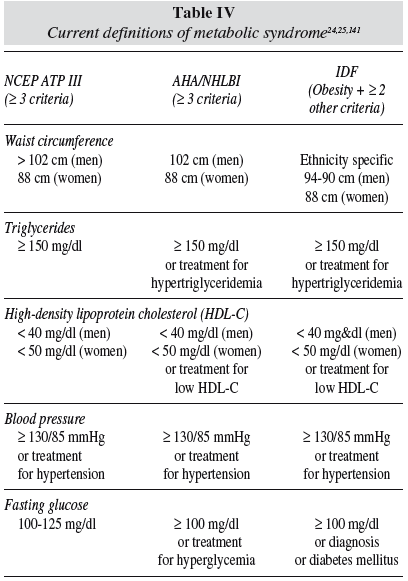
MetS is generally used to indicate a clinical situation in which different degrees of hypertension, impaired glucose tolerance, atherogenic dyslipidemia, central fat accumulation, as well as prothrombotic and proinflammatory states cluster together in the same individual. 22,23 Table IV reflects the different diagnosis criteria for MetS. Such a concurrence of disorders increases the probability of suffering from cardiovascular disease or T2DM, possibly more than what the sum of the single risk factors would predict. In 1998 the WHO24 published consensus definitions of MetS, based on serum values of triacylglycerols, HDL-cholesterol, blood pressure, and measurements of central obesity and fasting glucose. Later definitions increase accuracy of central obesity and insulin resistance,25 as presented in table IV.
During the last decade, the MetS has progressively become a major public health problem,26 both in healthy societies and in developing countries. Now, it is approaching epidemic proportions worldwide and its spreading prevalence is strictly associated with the adoption of a "westernized" lifestyle, which is characterized by lack of physical activity, excessive food intake, a combination of factors leading to overweight and obesity. In fact, obesity, and particularly visceral obesity, seems to be a major determinant of insulin resistance, hence preparing the path to the clustering of metabolic and non-metabolic factors embraced under the descriptive term of MetS.
The ability of body tissue to react to insulin becomes progressively more compromised as it moves maybe for ten to twenty years, through the stages of insulin resistance. During this stage of impaired insulin action, insulin secretion also becomes higher in an effort to correct the condition, until the beta cells of the pancreas are depleted and cease production, resulting in full-blown T2DM.27
Having reached this point, the already-known healthy properties of the n-3 fatty acids and their potential ones are of great interest to us.
N-3 fatty acids health effects
On September 8, 2004, the U.S. Food and Drug Administration gave "qualified health claim" status to EPA and DHA n-3 fatty acids, stating that "supportive but not conclusive research shows that consumption of EPA and DHA ω−3 fatty acids may reduce the risk of coronary heart disease". 27
N-3 fatty acids also exert some different good physiological actions, however several effects remain unclear, as is reflected in table V. The n-3 PUFA effects on glucose metabolism and its disorders will bereviewed thereafter.
Results from epidemiological and dietary intervention studies have shown that n-3 PUFA represents a class of powerfully bioactive compounds and that an adequate dietary intake of n-3 PUFA plays a critical role in human health in relation to non-communicable diseases.4
The effects of n-3 PUFA on plasma lipid levels havebeen documented in numerous studies.28 The lowering cholesterol effect in patients with established high cholesterol plasma levels has been reported although this effect is lower in healthy subjects which means it is health-status sensitive.29 The hypotriglyceridemic effect of n-3 fatty acids from diets rich in oily fish or fish oil supplements is well documented.30-33 Concerning the influence on serum cholesterol, the majority of studies found decreases in total cholesterol34-36 and increases in HDL-cholesterol32,37-39 though the effect on LDL-cholesterol is controversial.40 Several reports indicate that n-3 fatty acids do not change LDL-cholesterol, 41 others show an increase after consumption of high doses of n-3.32,38,39,42 A higher susceptibility of LDL particles to oxidation has been observed38,43 but not always44 in individuals given n-3 PUFA. This discrepancy should be related to the experimental conditions, fish intake vs n-3 supplement and doses used. Griffin et al39 compared 4 diets with an n-6/n-3 ratio between 5:1 and 3:1. The diets were enriched with ALA, EPA and DHA, or both. It was found that n3-PUFA (n-6/n-3 ratio of 3) decreased small dense-LDLs and postprandial triacylglycerols plasma levels, HDL-cholesterol did not change but HDL2 increased. Our group found that a dose of 0.8 g/day of fish oil induces the production of potentially less oxidized LDL in young women.45
In addition, the effects of n-3 fatty acids on serum lipids depend on the subject and whether the amount of SFA and MUFA in the diet are kept constant.1,46 A total of 1.5 to 3.5 g of n-3 PUFA intake per day is required to achieve a significant effect in hyperlipaemic patients (with variable intakes of SFA and MUFA). Reductions of the triacylglycerol levels of 25-40% can then be obtained.47,48
Södergren et al 49 compared in a randomized crossover study, the influence of a rapeseed oil-based diet (rich in ALA and oleic acid) and a SFA-rich diet on lipid levels and peroxidation in a group of moderately hyperlipidemic subjects. Total cholesterol, LDL-cholesterol and the LDL-cholesterol/HDL-cholesterol ratio were lower after the rapeseed oil diet, while HDLcholesterol and total triacylglycerol levels were similar after the two diets and there were no changes in the degree of lipid peroxidation. The authors attributed these results to the content of antioxidants in the rapeseed oil-diet and to other undetermined carry-over effects.
However, side effects, such as the association of oily fish consumption with mercury, lead and arsenic toxicity have also been reported. At present, fish traceability and safety are of great concern, but considering the risk/benefit ratio of fish consumption, the benefits are higher than the risks so public health recommendations include regular intake of these marine sources.50
The World Health Organization51 recommends population nutrient intake goals for n-3 PUFA to represent 1-2% of total energy intake,52,53 after consensus on its diet effects on chronic diseases. In agreement with this, Spanish guidelines establish a 0.5-1% of total energy to be ALA and 0.2-0.5% to be EPA plus DHA, within a n6/n3 ratio from 4 to 10.2,54
Effects of n-3 PUFA intake on insulin sensitivity
Fat level and type of fat
Few studies have addressed whether the fatty acid composition of the diet influences the fatty acid profile of skeletal muscle phospholipids and triacylglycerols. The fatty acid composition of the cell membrane is a dynamic system, and the regulation mechanisms are not fully understood. Both genetic55 and lifestyle-related factors, including diet56,57 and physical activity,58 seem to play a part in determining the fatty acid composition of skeletal muscle phospholipids. Table VI summarizes different studies which have observed n-3 PUFA and MUFA influence on insulin resistance, insulin sensitivity, and fasting plasma glucose levels indifferent type of populations.
Andersson et al59 investigated whether there is a correspondence between dietary fatty acid composition and fatty acid profile of skeletal muscle and triacylglycerols. They randomly assigned diets containing a high proportion of either SFA or MUFA (total fat, 36% of energy) to two groups of healthy men and women; one group started with a higher proportion of SFA than that of MUFA in the diet, and the other with the opposite (higher MUFA proportion in diet) for 3 months. Within each diet group, there was a second random assignment to supplementation with fish oil capsules containing 3.6 g n-3 PUFA/day (EPA and DHA) or placebo. A skeletal muscle biopsy sample was taken after the diet period, revealing that the fatty acid composition of skeletal muscle lipids reflects the fatty acid composition of the diet in healthy men and women.
This is important because skeletal muscle is an important tissue for the whole-body energy metabolism, including insulin-stimulated glucose uptake60 and fatty acid oxidation.61 The fatty acid composition of skeletal muscle phospholipids has been related to peripheral insulin sensitivity18,62-64 and obesity63,65 in several human populations. Figure 1 shows PUFA influence on skeletal-muscle membrane phospholipids. An increase in 20- and 22-carbon PUFA, ie, AA, EPA, and DHA, leads to increases in membrane fluidity, number of insulin receptors, and insulin action.66,67
Early work68 suggested a population-wide relationship between dietary fat (amount and quality) and obesity, diabetes mellitus and other degenerative diseases in which there is an increased affluence.
Epidemiological data suggest that subjects with higher intakes of fat are more prone to develop disturbances in glucose metabolism than subjects with lower intake of fat. Animal studies have given evidence that high-fat diets affect glucose metabolism negatively, but in humans (mostly T2DM patients) the results have been inconsistent and are likely confounded with differences in body weight.67 Greenland Inuits, who have a high total fat intake rich in PUFA (both n-3 and n-6), have surprisingly low prevalences of T2DM and of other glucose metabolism disorders.68,69 In studies in which the fatty acid composition of a high-fat diet has been modified to contain a higher proportion of unsaturated fat, an improvement of glucose metabolism, compared to a high-SFA diet, has been observed. Other studies46,70 using isocaloric diets with 44% of energy as total fat, confirm that the diet rich in SFA (PUFA/SFA ratio = 0.2) resulted in less favourable glucose tolerance, especially in subjects with type 4 hyperlipidemia (elevated triglyceride levels carried by VLDL). In normal subjects insulin concentrations, during the oral glucose tolerance test, were inversely associated with the dietary intake of PUFA and positively associated with the intake of SFA.71
Therefore, it seems that there are confounding factors in the human results. Among these, the overweight/obese condition mentioned above, drug therapy, and the dietary n-6 and n-3 imbalance should behig hlighted.
The hypothesis that a high background intake of n-6 PUFA could diminish beneficial effects of n-3 LCPUFA supplementation on insulin sensitivity was tested in Asian Indians living in the United Kingdom who used to have a significantly higher intake of PUFA, mainly as n-6 PUFA from vegetable oils, and a lower intake of n-3 LCPUFA than in whites.72 Volunteers received either a moderate or a high n-6 PUFA diet during a 6-week period, after which both groups were supplemented with 4.0 g fish oil/d (EPA+DHA) for an additional 6 weeks in combination with the dietary treatment. An insulin sensitivity test was performed after each of the 6 week dietary intervention periods; the result was that the background dietary n-6 PUFA concentration did not modulate the fish-oil supplementation measures of insulin sensitivity in this ethnic group. This finding should be explained considering that a 6 week-experimental period is enough to change membrane phospholipids, which is in agreement with most of the experimental periods used in lipid clinical assays.
Borkman et al18 determined the relationship between the fatty-acid composition of skeletal-muscle phospholipids and insulin sensitivity in patients undergoing coronary artery surgery in normal men. In both groups, skeletal muscle samples were obtained. They observed (determining fasting serum insulin levels and using the euglycaemic-clamp technique) that the more PUFA percentage of composition in the phospholipid fraction, the less insulin resistance, thus raising the possibility that changes in the fatty-acid composition of muscle modulate the action of insulin.51,73 Similarly, other authors63 in a study with adult male Pima Indians (a population with the highest reported incidence of T2DM in the world74) observed significant relationships between skeletal muscle membrane phospholipid fatty acid composition and both insulin action and adiposity, by determining insulin action (euglycaemic clamp method), percentage body fat, and muscle phospholipid fatty acid composition. Results revealed that insulin action was diminished as membrane unsaturation was low, and that insulin action is positively correlated with the percentage of C-20 and C-22 PUFA, including n-3 fatty acids.75,76 As indicated above, the fatty-acid composition of skeletal-muscle phospholipids reflects the profile of the fat ingested.
Improvement in insulin sensitivity after 12 weeks of n-3 fatty acids supplementation (1.3 g EPA and 2.9 g DHA) has been also observed in overweight and obese (BMI 24-44 kg/m2) premenopausal non-diabetic women.77 The combination of oily fish consumption with a weightreducing diet decreased more body weight than only the dietary regimen.48 In addition, n-3 fatty acids supplementation improved glucose and insulin metabolism in overweight patients treated for hypertension.39,48
In contrast, approximately 1 g/day of EPA plus DHA, using a food-based intervention, was unable to modify insulin sensitivity or postprandial lipase activity in men and women aged 45-70.39
In children younger than the age of 2, a significant inverse correlation between a fasting plasma glucose concentration and the percentage of LCPUFA in skeletal muscle membrane phospholipids has also been reported.56
The ingestion of both n-6 and n-3 fatty acids has been found to suppress hepatic lipogenesis, reduce the hepatic output of triglycerides, enhance ketogenesis, and induce fatty acid oxidation in both the liver and the skeletal muscle. Taken together, these effects might explain an actual improvement in glucose uptake and insulin sensitivity after n-3 (but also n-6) fatty acid ingestion. Insulin sensitivity may improve as a result of the effects of fatty acid intake on membrane fluidity.The improvement in glucose uptake after membrane enrichment with PUFA is apparently related to an increase in the residency time of glucose transporters type 1 and 4 (GLUT1 and GLUT4) in the plasma membrane (which leads to an expansion of the intracellular pool of glucose-6-phosphate and to increased skeletal muscle glycogen synthesis).78
There is controversy concerning the effects ofMUFA on insulin sensitivity. In fact, dietary protocols have often ignored the influence of MUFA modulating SFA, PUFA or specifically n-3 fatty acids actions. After comparing a group of healthy moderately hyperlipidemic subjects on a rapeseed oil (rich in ALA and oleic acid) diet, who were provided with 9 g/day of total n-3 fatty acids, with a SFA-rich diet during two consecutive 4 week periods separated by a 4 week wash-out period, lower fasting plasma glucose was observed but there were no changes in insulin and free fatty acids.49 This effect should be attributed not only to the relatively high dose of n-3 fatty acids in this study but also to the ~50% higher MUFA that the rapeseed oil provided. Nevertheless, in animal models, high n-3 fat intake favours glucose metabolism.79
Reinforcing the idea that MUFA improves insulin sensitivity, it was also observed that following consumption of three different diets for a 4 week-period in healthy men and women insulin sensitivity was clearly improved with a Mediterranean type diet, which is a relatively high fat diet rich in MUFA. Diets provided consisted of a SAT diet (38% total fat of which 12% MUFA), reduced fat diet (28% total fat of which 12% MUFA) and Mediterranean diet (38% total fat of which 22% MUFA).80
The KANWU study46 took also into account the MUFA interacting effect. Healthy adults randomly chosen were given a controlled isoenergetic diet containing either a high proportion of SFA or MUFA diet during 3 months. Within each group, there was a second assignment at random with a supplement of fish oil (3.6 g n-3 fatty acids/d) or placebo. Insulin sensitivity was significantly impaired on the SFA diet but did not change on the MUFA diet. Insulin secretion was not affected and the addition of n-3 fatty acids diet influenced neither insulin sensitivity nor insulin secretion. The favorable effects of substituting a MUFA diet by a saturated fatty acid diet on insulin sensitivity were only detected at a total fat intake below median, but were not noted in individuals with a high fat intake (> 37E%).
Our research group recently studied the effects of consuming five portions of oily fish or red meat during 2 months on insulin resistance in young women. The oily fish diet induced an increase in n-3 intake reaching 3 g/day, without changes in SAT fat and a slight reduction in MUFA intake (that was around 50% total fat). Under these experimental conditions the oily fish diet induced a significant decrease in insulin levels and insulin resistance (determined by HOMA and QUICKI).81
Clinical studies in diabetic subjects
Early studies, such as the one performed by Jensen et al82 with type 1 diabetes patients receiving cod-liver oil (natural source rich in n-3 PUFA), showed improvement in complications attributable to microvascular disease but blood glucose concentrations were unchanged.
Some studies have been conducted on the effects of n-3 PUFA in patients with T2DM.83 In most of them, fish-oil consumption rose plasma glucose concentrations. In many of these studies, however, the number of subjects was small, the dose of n-3 fatty acids was > 3g/day and controls were insufficient.
In a randomized, double-blind, placebo-controlled, crossover trial, patients with T2DM consumed 6 g/day of n-3 fatty acids (EPA plus DHA) for 6 months in addition to their usual oral therapy.84 Fasting serum glucose concentrations increased 11% during the n-3 PUFA phase and 8% during the placebo phase (olive oil). Thus a non-significant net glucose increase of 3% was observed. This study showed convincingly that n-3 PUFA intake, along with oral therapy for diabetes, has no adverse effects on glycaemic control.
The review by De Caterina et al19 concluded that the administration of n-3 fatty acids does not apparently affect glucose control in patients with type 1 diabetes but it is notable that the studies they reviewed included a relatively low number of patients and were of short duration.
Another review article85 based on very few observations stated that in patients with T2DM n-3 fatty acids had an adverse effect on metabolic control of diabetes, but more recent studies report no adverse effect of dietary n-3 fatty acids on glucose control. The study by Sirtori et al86 is of special interest because of the relatively high number of patients treated, and its duration (1 year). Eighty nine patients with T2DM were randomized to EPA plus DHA 2.6 g/day for the first 2 months and then 1.7 g/day for the next 4 months. Olive oil control was used as placebo. Treatment was then continued in a non-blind manner up to 1 year. No significant differences were observed between the treatment groups in relation to fasting glucose or insulin levels. Supplementation with 1.7 g/day to all patients for a further 6 months produced no deterioration of glucose control after 1 year of treatment. In this study, there were, however, patients in whom metabolic control deteriorated during treatment with n-3 fatty acids, but whether this was caused by n-3 fatty acids or was partof the natural history of the disease is uncertain.
Delarue et al87 observed that in patients with T2DM, fish oil dietary supplementation fails to reverse insulin resistance for unclear reasons and conversely, in healthy humans, fish oil has many physiological effects, indeed, it reduces insulin response to oral glucose without altering the glycaemic response. Later Mostad et al88 studied the effects of fish oil supplements on metabolic variables in subjects with T2DM by studying short-term (1 week) and longer-term (9 weeks) effects of n-3 fatty acids. They provided T2DM nonhypertriacylglycerolemic subjects 1.8 g EPA, 3.0 g DHA, and 5.9 g total n-3 PUFA in the form of fish oil. The control group received a similar amount of corn oil/day (8.5 g LA). Glucose concentrations (homemonitorized) were ≈1 mmol/L higher in the fish oilgroup than in the corn oil group at the end of the intervention. Glucose utilization measured by using an isoglycemic clamp technique was lowered in the fish oil group compared with that in the corn oil group at the end of the intervention, concluding that a high intake of fish oil moderately increases blood glucose and decreases insulin sensitivity in T2DM subjects in a timedependent manner.
Historically, epidemiologic studies have reported a lower prevalence of impaired glucose tolerance and T2DM populations consuming large amounts of the n-3 LCPUFA found mainly in fish.89
Several clinical studies in the 1980s and early 1990s reported adverse effects on blood glucose control and insulin activity in subjects with T2DM who consumed large amounts of fish.86,90,91 It is now believed that these deleterious effects were largely attributable to the high doses used (e.g. 10 g/day fish oil or more). Recent studies using low doses of n-3 LC-PUFA, ranging from 1 to 2 g/day, have reported no deterioration of glucose control.92-94 In addition, one study shows that the increase in glycated hemoglobin associated with daily fish consumption providing 3.6 g/day n-3 PUFA could be prevented by moderate exercise (55% to 65% maximum oxygen consumption/min).95,96
Epidemiological studies
Stene et al97 performed an epidemiological study in Norwegian pregnant women. They discovered that the use of cod liver oil during pregnancy was associated with lower risk of Type I diabetes in the offspring. This effect was found only in mothers taking cod liver oil, not in mothers taking multivitamin supplements.
Nevertheless, pregnant women should be wary and not consume large amounts, since researchers in Iceland report that a high intake of cod liver oil is associated with a nearly five-fold increased risk of gestational hypertension98, although this study did not control for mercury, which can be present in harmful amounts in fish, and which is another cause of hypertension and cardiovascular disease.99,100
T2DM was less prevalent among Japanese islanders compared to their mainland counterparts.100 Lower prevalence was attributed mainly to diets rich in n-3 LCPUFA. The incidence of T2DM has increased rapidly among native and migrant populations, as much as 80% among Alaskan natives.101 The increase has been associated with the greater consumption of non-indigenous foods, changes in lifestyle, and fatty acid imbalance.102,103
In the Finnish and Dutch cohorts of the Seven Countries Study, fish consumption was inversely related to 2-hour glucose levels during a 20-year follow-up of male participants.71 Fish consumption was associated with reduced risk of developing impaired glucose tolerance.104
In Iceland, despite the high prevalence of overweight people and obesity, the prevalence of T2DM is lower than in other Nordic countries. Thorsdottir et al105 reported that the prevalence of T2DM in Icelandic men was inversely associated with both the n-3 PUFA and EPA content of milk, and positively associated with the ratio of n-6/n-3 fatty acids in milk. Icelandic milk contains significantly more n-3 LC-PUFA than milk in other Nordic countries (0.22 ± 0.05% vs 0.06 ± 0.01% for Norway and 0.03 ± 0.01% for Denmark), mainly because animal fodder contains fish meal. Icelandic milk is also lower in n-6 PUFA. Daily consumption of 500 mL Icelandic milk for a week provides about the same amount of EPA as one 100-g serving of sea trout (176 vs 165 mg EPA). In this population, the unique composition of dairy fat could be protective against T2DM.
By contrast, in a study of nearly 36,000 older Iowa women who did not have T2DM at enrolment, diabetes incidence after 11 years was positively associated with n-3 LC-PUFA consumption.74,106 After adjusting for other dietary fat, only vegetable fat was related to diabetes risk and appeared protective.
Van Dam et al107 reported in the US Health Professional Follow-Up Study that n-3 LC-PUFA intake was not associated with the risk of diabetes in more than 42,000 men, but total and saturated fat intake increased risk. Promising preliminary evidence suggests that the development of T2DM can be retarded or prevented by increased consumption of n-3 LC-PUFA and reduced consumption of SFA as was reported by Ebbeson.103,108
Nutrigenomic aspects
The sequential steps preceding T2DM: failure of insulin action at the cells, compensatory increase in insulin secretion, hyperglycaemia, decreased ß-cell function, and the concomitant cascade of metabolic alterations, are all the result of polygenic and environmental factors. In addition, since ardiovascular disease and obesity are also multigenic and multifactorial pathologies, we are far from understanding the genetic-diet interaction behind the MetS, particularly in relation to the interacting influence of the different PUFA and in particular of n3 fatty acid intakes. Nevertheless, here we briefly present the genetic variants that have been associated with insulin resistance and the possible link with fat intake.
Genes related to transport of insulin have been investigated. A case in point is the insulin receptor substrate (IRS) that plays a critical role in hepatic insulin signalling and expression of genes involved in gluconeogenesis, glycogen synthesis and lipid metabolism,109 and the insulin-responsive GLUT4, that plays a key role in glucose uptake and metabolism in insulin target tissues.110 However, no conclusive results are available concerning specific polymorphisms of these genes and higher risk of insulin resistance, T2DM or the MetS.
Other candidate genes are adiponectin and the adiponectin receptors. Adiponectin is an adipokine secreted by adipocytes that enhances insulin sensitivity and has antiinflammatoryaction. In skeletal muscle, it induces glucose uptake and energy expenditure, while in liver it reduces glucose uptake and fat accumulation, thus it protects against MetS. There are many reports concerning the metabolic effects of variation in the gene coding for adiponectin, named ADIPOQ. Meta-analyses of these studies support the hypothesis that variability in this gene contributes to the modulation of circulating adiponectin levels and the risk of insulin resistance and CVD. Two linkage disequilibrium blocks have been identified: in the 5' block, the g.-11391G --> A variant has modest but significant influence on adiponectinaemia, in the 3' block, the g.+276G --> T variant is a strong determinant of insulin resistance and CVD though only marginally significant for adiponectin levels. In contrast, results in relation to genetic variants in the adiponectin receptor gene are still very limited and inconclusive.111
Interestingly, serum levels of adiponectin can be modulated by peroxisome proliferator-activated receptor (PPAR)-γ agonists. Polymorphisms in PPARs isoforms may be among the most important single-gene contributors to dyslipidemias, insulin resistance, and T2DM.112
PPARs are central regulators of lipoprotein metabolism and glucose homeostasis that are considered particularly useful for improving glycaemic control and comorbidities in patients with T2DM.113 There are three types: PPAR-α, PPAR β/δ, and PPAR-γ. Clinical trials of PPAR-α;114 agonists have demonstrated efficacy in reducing cardiovascular events; however, these benefits have been confined to subgroups of patients with low levels of HDL-cholesterol or high levels of triacylglycerols, so future studies of PPAR-γ agonists or dual PPAR-α/γ agonists require further delineation of the risk profile to avoid adverse outcomes in susceptible patients. PPAR-γ are nuclear receptors that positively modulate insulin sensitivity.115
The finding that PPAR-γ are molecular targets of thiazolidinediones (TZDs), orally active insulin-sensitizing agents increasingly used for treatment of T2DM, represents a new step in knowledge of insulin resistance linked to cardiovascular diseases.116-118 Adipose tissue and skeletal muscle appear to be the key targets for TZDs.119-122 TZD activation of PPAR-γ in skeletal muscle is associated with increased expression of lipoprotein lipase and GLUT4.78,110 In this context, several studies have shown that treatment with TZDs increases adiponectin, rising the hypothesis that some of the anti-diabetic action of these drugs are due to the effects on adiponectin levels.
The influence of PPAR-γ polymorphisms on fat intake response has been investigated.123-125 Cardona et al,123 studied postprandial lipid metabolism in subjects classified according to their PPAR-γ and APOE genotypes, the corresponding genes being named PPARG and APOE, respectively. A variant of PPARG, PPARG2, contains an amino substitution of proline for alanine at codon 12 (Pro12Ala). This variant together with a non-E3/E3 APOE genotype was associated with a high risk for postprandial hypertriglyceridemia in patients with the MetS. It was observed that the Ala12 sequence variant is associated with a worse metabolic profile than Pro12 in relation to PPAR-γ expression and oxidative imbalance after the fat load.124
Recent genome-wide association studies have provided an important resource for furthering our understanding of T2DM disease mechanisms. Genes previously unsuspected of playing a role in diabetes are now implicated in the disease process. These include genes in cellcycling control (CDKN2A/2B, CDKAL1), transcription factors (TCF7L2, HHEX), and ion channels (SLC30A8). Two common variants alter diabetes risk through a primary effect on obesity. These variants are all associated with insulin-secretory defects in the general population and show little if any relationship to insulin esistance.126
PUFA and n-3 PUFA at high doses may increase oxidative stress, which may interact with diverse gene variants, as indicated above. Also n-3 PUFA are agonists of PPAR-γ, and this mechanism may explain some of the effects of n3 on insulin sensitivity. Therefore, research into the response of individuals presenting the Ala12 or Pro12 of the PPARG2 to dietary n-3 LC-PUFA, or n-3 PUFA supplements is highly advisable. Similar nutrigenomic interaction should be explored as new polymorphisms are demonstrated to be implicated in the MetS.
Conclusions
The use of n-3 fatty acids should be considered within more global strategies, which include changes in lifestyle, such as adhering to a healthy Mediterranean type of diet and doing regular physical exercise. Reduction of dietary SFA and its partial replacement by MUFA and PUFA, including n-3 family, leads to an improvement in insulin sensitivity and associated metabolic abnormalities. There has been concern in the past about a possible deterioration in glucose homeostasis after intake of n-3 fatty acids in patients with T2DM, but these have been generally observed after using very high doses of n-3 PUFA supplements and the possible interacting effect of mercury ingestion has not been considered. Fish oil, a natural source of n-3 PUFA, has many physiological effects, indeed, it reduces insulin response to oral glucose without altering the glycaemic response in healthy humans; this is encouraging in the perspective of prevention of insulin resistance but further clinical and basic studies must be designed to confirm and complete our knowledge in this field. Finally, the nutrigenomic viewpoint should be included in all studies.
References
1. Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr 1991; 54: 438-463. [ Links ]
2. Sánchez-Muniz FJ, Bastida S. Biodisponibilidad de ácidos grasos. Rev Nutr Pract 2000; 4: 48-64. [ Links ]
3. Herrera E. Metabolismo del tejido adiposo y sensibilidad insulínica en la gestación. In: Vaquero MP, ed. Genética, nutrición y enfermedad. Consejo Superior de Investigaciones Científicas, Instituto Tomás Pascual Sanz, Edimsa, pp. 147-156, Madrid, 2008. [ Links ]
4. Li D. Omega-3 fatty acids and non-communicable diseases. Chin Med J (Engl) 2003; 116 (3): 453-458. [ Links ]
5. Dupont JL. Fats and oils. In: Sadler MJ, Strain JJ, Caballero B, eds. Encyclopedia of Human Nutrition, Academic Press, vol.2, pp. 719-721, London, 1998. [ Links ]
6. González Deschamps E, Palmeros Exsome C, Villanueva Sánchez J, Torres Flores B, Bastida S, Vaquero MP, Sánchez-Muniz FJ: Metabolic syndrome prevalence and its association with the body mass index in university students. Med Clin (Barc) 2007; 129 (20): 766-769. [ Links ]
7. Mataix J. Lípidos alimentarios. In: Mataix J, Gil A, eds. Libro blanco de los omega-3. Los ácidos grasos poliinsaturados omega 3 y monoinsaturados tipo oleico y su papel en la salud, Puleva Food Editions, pp. 17-19, Granada, 2004. [ Links ]
8. Sánchez-Muniz FJ. Los lípidos. In: García Arias MT, García Fernández MC, eds. Nutrición y dietética, Universidad de León, pp. 119-133, León, 2003. [ Links ]
9. Lobb K, Chow CK. Fatty acid classification and nomenclature. In: Chow CK, ed. Fatty acids in foods and their health implications, 3rd edition, CRC Press Taylor and Francis Group, pp. 3-5, Boca Ratón, 2008. [ Links ]
10. Whelan J, Rust C. Innovative dietary sources of n-3 fatty acids. Annu Rev Nutr 2006; 26: 75-103. [ Links ]
11. Haag M, Dippenaar NG. Dietary fats, fatty acids and insulin resistance: short review of a multifaceted connection. Med Sci Monit 2005; 11 (12): RA359-367. [ Links ]
12. Menzaghi C, Trischitta V, Doria A. Genetic influences of adiponectin on insulin resistance, type 2 diabetes, and cardiovascular disease. Diabetes 2007; 56: 1198-1209. [ Links ]
13. Dib, SA. Insulin resistance and metabolic syndrome in type 1 diabetes mellitus. Arq Bras Endocrinol Metab 2006; 2 (50): 250-263. [ Links ]
14. Bhoraskar A. Nutrition in prediabetes. J Indian Med Assoc 2006; 103: 596-599. [ Links ]
15. Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between Type I and Type II diabetes. Diabetología 2001; 44: 914-922. [ Links ]
16. Yaney GC, Corkey BE. Fatty acid metabolism and insulin secretion in pancreatic beta cells. Diabetología 2003; 46: 1297-1312. [ Links ]
17. Benito P, Caballero J, Moreno J, Gutierrez-Alcantara C, Muñoz C, Rojo G et al. Effects of milk enriched with omega-3 fatty acid, oleic acid and folic acid in patients with metabolic syndrome. Clin Nutr 2006; 25: 581-587. [ Links ]
18. Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med 1993; 328: 238-244. [ Links ]
19. De Caterina R, Madonna R, Bertolotto A, Schmidt EB. n-3 Fatty acids in the treatment of diabetic patients. Biological rationale and clinical data. Diabetes Care 2007; 30: 1012-1126. [ Links ]
20. Serrano Ríos M, Caro JF, Carraro R, Gutiérrez Fuentes JA. Introduction. In: Serrano Ríos M, Caro JF, Carraro R, Gutiérrez Fuentes JA, editors. The Metabolic Syndrome at the beginning of the XXIst century. A genetic and molecular approach. Madrid: Elsevier España; 2005, pp. 1-2. [ Links ]
21. Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr 2004; 23: 447-456. [ Links ]
22. Crepaldi G. Origin and development of the Metabolic Syndrome. In: Serrano Ríos M, Caro JF, Carraro R, Gutiérrez Fuentes JA, eds. The Metabolic Syndrome at the beginning of the XXIst century. A genetic and molecular approach. Elsevier España, pp. 5-12. Madrid, 2005. [ Links ]
23. Ruiz-Torres A. Aging and the Metabolic Syndrome. In: Serrano Ríos M, Caro JF, Carraro R, Gutiérrez Fuentes JA, eds. The Metabolic Syndrome at the beginning of the XXIst century. A genetic and molecular approach. Elsevier España, pp. 83-91. Madrid, 2005. [ Links ]
24. Alberti K, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1, diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539-553. [ Links ]
25. Stolar, M. Metabolic syndrome: controversial but useful. Cleve Clin J Med 2007; 3 (74): 199-208. [ Links ]
26. Lorenzo C, Serrano Ríos M. Epidemiology of the Metabolic Syndrome. In: Serrano Ríos M, Caro JF, Carraro R, Gutiérrez-Fuentes JA, eds. The Metabolic Syndrome at the beginning of the XXIst century. A genetic and molecular approach. Elsevier España; 2005, pp. 107-123, Madrid, 2005. [ Links ]
27. http://www.who.int/nutrition/topics/5_population_nutrient/en/index13.html. [ Links ]
28. Harris WS, Bulchandani. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol 2006; 17 (4): 387-393. [ Links ]
29. Lichtenstein AH. Nutritional management of hyperlipidaemia. In: Sadler MJ, Strain JJ, Caballero B, editors. Encyclopedia of Human Nutrition, Academic Press, vol. 2, pp. 1037-1038, London, 1998. [ Links ]
30. Carpentier YA, Portois L, Malaisse WJ. n-3 fatty acids and the metabolic syndrome. Am J Clin Nutr 2006; 83 (6 Supl.): 1499S-1504S. [ Links ]
31. Turley E, Strain JJ. Fish oils, eicosanoid biosynthesis and cardiovascular disease: an overview. Int J Food Sci Nutr 1993; 44: 145-152. [ Links ]
32. Nestel PJ. Effects of n-3 fatty acids on lipid metabolism. Annu Rev Nutr 1990; 10: 149-167. [ Links ]
33. Sayago-Ayerdi SG, Vaquero MP, Schultz-Moreira A, Bastida S, Sánchez-Muniz FJ. Utilidad y controversias del consumo de ácidos grasos de cadena media sobre el metabolismo lipoprotéico y obesidad. Nutr Hosp 2008; 23 (3): 265-276. [ Links ]
34. Mozaffarian D. Effects of dietary fats versus carbohydrates on coronary heart disease: a review of the evidence. Curr Atheroscler Rep 2005; 7 (6): 435-445. [ Links ]
35. Rivellese AA, Lilli S. Quality of dietary fatty acids, insulin sensitivity and type 2 diabetes. Biomed Pharmacother 2003; 57 (2): 84-87. [ Links ]
36. Davidson MH. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am J Cardiol 2006; 98 (4A): 27i-33i. [ Links ]
37. Samaha FF. Effect of very high-fat diets on body weight, lipoproteins, and glycemic status in the obese. Curr Atheroscler Rep 2005; 7 (6): 412-420. [ Links ]
38. Finnegan YE, Howarth D, Minihane AM, Kew S, Miller GJ, Calder PC et al. Plant and marine derived (n-3) polyunsaturated fatty acids do not affect blood coagulation and fibrinolytic factors in moderately hyperlipidemic humans. J Nutr 2003; 133 (7): 2210-2213. [ Links ]
39. Griffin MD, Sanders T, Davies IG, Morgan LM, Millward DJ, Lewis F et al. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on insulin sensitivity, lipoprotein size, and postprandial lipemia in men and postmenopausal women aged 45-70 y: the OPTILIP Study. Am J Clin Nutr 2006; 84: 290-1298. [ Links ]
40. Sanders TA. Effects of unsaturated fatty acids on blood clotting and fibrinolysis. Curr Opin Lipidol 1996; 7: 20-23. [ Links ]
41. Leigh-Firbank EC, Minihane AM, Leake DS, Wright JW, Murphy MC, Griffin BA, Williams CM. Eicosapentaenoic acid and docosahexaenoic acid from fish oils: differential associations with lipid responses. Br J Nutr 2002; 87: 435-445. [ Links ]
42. Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002; 106: 2747-2757. [ Links ]
43. Pedersen H, Petersen M, Major-Pedersen A, Jensen T, Nielsen NS, Lauridsen ST, Marckmann P. Influence of fish oil supplementation in vivo and in vitro oxidation resistance of low-density lipoprotein in type 2 diabetes. Eur J Clin Nutr 2003; 57: 713-720. [ Links ]
44. Higgins S, Carroll YL, McCarthy SN, Corridan BM, Roche HM, Wallace JM et al. Susceptibility of LDL to oxidatuive modification in healthy volunteers supplemented with low doses of n-3 polyunsaturated fatty acids. Br J Nutr 2001; 85: 23-31. [ Links ]
45. Sánchez-Muniz FJ, Bastida S, Viejo JM, Terpstra AHM. Small supplements of n-3 fatty acids change serum low density lipoprotein composition by decreasing phospholipid and apolipoprotein B concentrations in young adult women. Eur J Nutr 1999; 1: 20-37. [ Links ]
46. Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU study. Diabetología 2001; 44: 312-319. [ Links ]
47. Wahrburg U. What are the health effects of fat? Eur J Nutr 2004 (Supl. 1) 43: I/6-I/11. [ Links ]
48. Mori TA, Bao DQ, Burke V, Puddey IB, Watts GF, Beilin L. Dietary fish as a major component of a weight-loss diet: effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am J Clin Nutr 1999; 70: 817-825. [ Links ]
49. Södergren E, Gustafsson IB, Basu S, Nourooz-Zadeh J, Nälsén C, Turpeinen A et al. A diet containing rapeseed oil-based fats does not increase lipid peroxidation in humans when compared to a diet rich in saturated fatty acids. Eur J Clin Nutr 2001; 55: 922-931. [ Links ]
50. Wang C, Chung M, Lichtenstein A, Balk E, Kupelnick B, DeVine D, et al. Effects of Omega-3 fatty acids on cardiovascular disease. Evidence report/ Technology assessment No. 94 (Prepared by Tufts-New England Medical Center Evidencebased Practice Center, under Contract No. 290-02-0022). AHRQ Publication No. 04-E009-2. Rockville, MD: Agency for Healthcare Research and Quality. March 2004. [ Links ]
51. http://www.who.int/nutrition/topics/4_dietnutrition_prevention/en/index4.html. [ Links ]
52. Fats and oils in human nutrition. Report of a Joint FAO/WHO Expert Consultation. Rome, Food and Agriculture Organization of the United Nations. 1994 (FAO Food and Nutrition Paper, No. 57). [ Links ]
53. Preparation and use of food-based dietary guidelines. Report of a Joint FAO/WHO Consultation. Geneva, World Health Organization. 1998 (WHO Technical Report Series, No. 880). [ Links ]
54. Ros E. Guía para una alimentación cardiosaludable. Aporte de grasa. In: Sociedad Española de Nutrición Comunitaria. Guías alimentarias para la población española. Recomendaciones para una dieta saludable. IM & C; pp. 413-421, Madrid, 2001. [ Links ]
55. Baur LA, O'Connor J, Pan DA, Storlien LH. Relationships between maternal risk of insulin resistance and the child's muscle membrane fatty acid composition. Diabetes 1999; 48: 112-116. [ Links ]
56. Baur LA, O'Connor J, Pan DA, Kriketos AD, Storlien LH. The fatty acid composition of skeletal muscle membrane phospholipid: its relationship with the type of feeding and plasma glucose levels in young children. Metabolism 1998; 47: 106-112. [ Links ]
57. Baur LA, O'Connor J, Pan DA, Wu BJ, O'Connor MJ, Storlien LH. Relationships between the fatty acid composition and erythrocyte membrane phospholipid in young children and the effect of type of infant feeding. Lipids 2000; 35: 77-82. [ Links ]
58. Andersson A, Sjödin A, Olsson R, Vessby B. Effects of physical exercise on phospholipid fatty acid composition in skeletal muscle. Am J Physiol Endocrinol Metab 1998; 274: E432-8. [ Links ]
59. Andersson A, Nälsén C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am J Clin Nutr 2002; 76: 1222-1229. [ Links ]
60. DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981; 30: 1000-1007. [ Links ]
61. Cortright RN, Muoio DM, Dohm GL. Skeletal muscle lipid metabolism: a frontier for new insights into fuel homeostasis. Nutr Biochem 1997; 8: 228-245. [ Links ]
62. Clore JN, Li J, Gill R et al. Skeletal muscle phosphatidylcholine fatty acids and insulin sensitivity in normal humans. Am J Physiol Endocrinol Metab 1998; 275: E665-670. [ Links ]
63. Pan DA, Lillioja S, Milner MR et al. Skeletal muscle membrane lipid composition is related to adiposity and insulin action. J Clin Invest 1995; 96: 2802-2808. [ Links ]
64. Vessby B, Tengblad S, Lithell H. Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia 1994; 37: 1044-1050. [ Links ]
65. Kriketos AD, Pan DA, Lillioja S, et al. Interrelationships between muscle morphology, insulin action, and adiposity. Am J Physiol Regul Integr Comp Physiol 1996; 270: R1332-1339. [ Links ]
66. Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr 1999; 70 (Supl.): 560S-569S. [ Links ]
67. Lichtenstein AH, Schwab US. Relationship of dietary fat to glucose metabolism. Atherosclerosis 2000; 150: 227-243. [ Links ]
68. Deutch B, Dyerberg J, Pedersen HS, Asmund G, Møller P, Hansen JC. Traditional and modern Greenlandic food. Dietary composition, nutrients and contaminats. Dietary composition and contaminants in north Greenland, in the 1970s and 2004. Sci Total Environ 2007; 384: 106-119. [ Links ]
69. Jørgensen ME, Borch-Johnsen K, Bjerregaard P. Lifestyle modifies obesity-associated risk of cardiovascular disease in a genetically homogeneous population. Am J Clin Nutr 2006; 84: 29-36. [ Links ]
70. Vessby B, Gustafsson IB, Boberg J, Karlström B, Lithell H, Werner I. Substituting polyunsaturated for saturated fat as a single change in a Swedish diet: effects on serum lipoprotein metabolism and glucose tolerance in patients with hyperlipoproteinaemia. Eur J Clin Invest 1980; 10: 193-202. [ Links ]
71. Feskens EJM, Loeber JG, Kromhout D. Diet and physical activity as determinants of hyperinsulinemia: The Zutphen Elderly Study. Am J Epidemiol 1994; 140: 350-360. [ Links ]
72. Brady LM, Lovegrove SS, Lesauvage S, Gower BA, Minihane AM, Williams CM et al. Increased n-6 polyunsaturated fatty acids do not attenuate the effects of long-chain n-3 polyunsaturated fatty acids on insulin sensitivity or triacylglycerol reduction in Indian Asians. Am J Clin Nutr 2004; 79: 983-991. [ Links ]
73. http://www.who.int/ictrp/who_role.pdf. [ Links ]
74. Steyn NP, Mann J, Bennett PH, Temple N, Zimmet P, Tuomilehtoet al. Diet, Nutrition and the prevention of type 2 diabetes. Public Health Nutr 2004; 7(1A): 147-165. http://www.who.int/ictrp/who_role.pdf. [ Links ]
75. Storlien LH, Pan DA, Kriketos AD et al. Skeletal muscle membrane lipids and insulin resistance. Lipids 1996: 31 (Supl.): S261-265. [ Links ]
76. Storlien LH, Jenkins AB, Chisholm DJ, Paseoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationships to muscle tryglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes 1991; 40: 280-289. [ Links ]
77. Browning LM, Krebs JK, Moore CS, Mishra JD, O'Connell MA, Jebb SA. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab 2007; 9: 70-80. [ Links ]
78. Nugent C, Prins JB, Whitehead JP, Wentworth JM, Chatterjee VK, O'Rahilly S. Arachidonic acid stimulates glucose uptake in 3T3-L1 adipocytes by increasing GLUT1 and GLUT4 levels at the plasma membrane. Evidence for involvement of lipoxygenase metabolites and peroxisome proliferator-activated receptor gamma. J Biol Chem 2001; 276 (12): 9149-9157. [ Links ]
79. Browning LM. n-3 Polyunsaturated fatty acids, inflammation and obesity-related disease. P Nutr Soc 2003; 62: 447-453. [ Links ]
80. Pérez-Jiménez, F, López-Miranda J, Pinillos MD, Gómez P, Paz-Rojas E, Montilla P, Marín C et al. A Mediterranean and a high-carbohydrate diet improve glucose metabolism in healthy young persons. Diabetología 2001; 44: 2038-2043. [ Links ]
81. Navas-Carretero S, Pérez-Granados AM, Schoppen S, Vaquero MP. An oily fish diet increases insulin sensitivity compared to a red meat diet in young iron deficient women. Br J Nutr 2009; doi: 10.10.1017/50007114509220794. [ Links ]
82. Jensen T, Stender S, Goldstein K, Holmer G, Deckert T. Partial normalization by dietary cod-liver oil of increased microvascular albumin leakage in patients with insulin-dependent diabetes and albuminuria. N Engl J Med 1989; 321: 1572-1577. [ Links ]
83. Harris WS. 1996. Do ω-3 fatty acids worsen glycemic control in NIDDM? ISSFAL Newsletter 1996; 3: 6-9. [ Links ]
84. Connor WE, Prince MJ, Ullmann D et al. The hypotriglyceridemic effect of fish oil in adult-onset diabetes without adverse glucose control. Ann NY Acad Sci 1993; 683: 337-340. [ Links ]
85. Heine RJ. Dietary fish oil and insulin action in humans. Ann NY Acad Sci 1993; 683: 10-21. [ Links ]
86. Sirtori CR, Crepaldi G, Manzato E, Mancini M, Rivellese A, Paoletti R et al. One-year treatment with ethyl esters of n-3 fatty acids in patients with hypertriglyceridemia and glucose intolerance: reduced triglyceridemia, total cholesterol and increased HDL-D without glycemic alterations. Atherosclerosis 1998; 137: 419-427. [ Links ]
87. Delarue J, LeFoll C, Corporeau C, Lucas D. n-3 Long chain polyunsaturated fatty acids: a nutritional tool to prevent insulin resistance associated to type 2 diabetes and obesity? Reprod Nutr Dev 2004; 44: 289-299. [ Links ]
88. Mostad IL, Bjerve S, Bjorgaas MR, Lydersen S, Grill V. Effects of n-3 fatty acids in subjects with type 2 diabetes: reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am J Clin Nutr 2006; 84: 540-550. [ Links ]
89. Nettleton JA, Katz R. n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc 2005; 105: 428-440. [ Links ]
90. Borkman M, Chisholm DJ, Furler SM, Storlien LH, Draegen EW, Simons LA et al. Effects of fish oil supplementation on glucose and lipid metabolism in NIDDM. Diabetes 1989; 38: 1314-1319. [ Links ]
91. Vessby B. n-3 fatty acids and blood glucose control in diabetes mellitus. J Int Med Suppl 1989; 225: 207-210. [ Links ]
92. Westerveld HT, de Graaf JC, van Breugel HH, Akkerman JW, Sixma JJ, Erkelens DW et al. Effects of low-dose EPA-E on glycemic control, lipid profile, lipoprotein (a), platelet aggregation, viscosity, and platelet and vessel wall interaction in NIDDM. Diabetes Care 1993;16: 683-638. [ Links ]
93. Axelrod L, Camuso J, Williams E, Kleinman K, Briones E and Scoenfeld D. Effects of a small quantity of omega-3 fatty acids on cardiovascular risk factors in NIDDM. A randomized prospective, double-blind, controlled study. Diabetes Care 1994; 17: 37-44. [ Links ]
94. Luo J, Rizkalla SW, Vidal H, Oppert JM, Colas C, Boussairi A et al. Moderate intake of n-3 fatty acids for 2 months has no detrimental effect on glucose metabolism and could ameliorate the lipid profile in type 2 diabetic men. Results of a controlled study. Diabetes Care 1998; 21: 717-724. [ Links ]
95. Dunstan DW, Mori TA, Puddey IB, Beilin LJ, Burke V, Morton AR et al. The independent and combined effect of aerobic exercise and dietary fish intake on serum lipids and glycemic control in NIDDM. A randomized controlled study. Diabetes Care 1997; 20: 913-921. [ Links ]
96. Bathena SJ. Fatty acid metabolism in diabetes. In: Chow CK, editor. Fatty acids in foods and their health implications, 3rd ed., CRC Press Taylor and Francis Group, pp. 1152-1173. Boca Ratón, 2008. [ Links ]
97. Stene LC, Ulriksen J, Magnus P et al. Use of cod liver oil during pregnancy associated with lower risk of Type I diabetes in the offspring. Diabetología 2000; 43: 1093-1098. [ Links ]
98. Olafsdottir AS, Skuladottir GV, Thorsdottir I, Hauksson A, Thorgeirsdottir H, Steingrimsdottir L. Relationship between high consumption of marine fatty acids in early pregnancy and hypertensive disorders in pregnancy. Br J Obstet Gynaecol 2006; 113: 301-309. [ Links ]
99. Delarue J, Li CH, Cohen R, Corporeau C, Simon B. Mercury as a risk factor for cardiovascular diseases. J Nutr Biochem 2007; 18: 75-85. [ Links ]
100. Kagawa Y, Nishizawa M, Suzuki M, Miyatake T, Hamamoto T, Goto K et al. Eicosapolyenoic acids of serum lipids of Japanese islanders with low incidence of cardiovascular diseases. J Nut Sci Vitaminol (Tokyo) 1982; 28: 441-453. [ Links ]
101. Schraer CD, Mayer AM, Vogt AM, Naylor J, Brown TM, Hastie J et al. The Alaska Native diabetes program. Int J Circumpolar Health 2001; 60: 487-494. [ Links ]
102. Murphy NJ, Schraer CD, Thiele MC, Boyko EJ, Bulkow LR, Doty BJ et al. Dietary change and obesity associated with glucose intolerance in Alaska Natives. J Am Diet Assoc 1995; 95: 676-682. [ Links ]
103. Ebbesson SO, Kennish J, Ebbesson L, Go O, Yeh J. Diabetes is related to fatty acid imbalance in Skimos. Int J Circumpolar Health 1999; 58: 108-119. [ Links ]
104. Vilgbersson S, Sigurdsson G, Sigvaldason H, Hreidarsson AB, Sigfusson N. Prevalence and incidence of NIDDM in Iceland: Evidence for stable incidene among males and females 1967-1991-the Reikjavik Study. Diabet Med 1997; 14: 491-498. [ Links ]
105. Thorsdottir I, Hill J, Ramel A. Omega-3 fatty acid supply from milk associates with lower type 2 diabetes in men and coronary heart disease in women. Prev Med 2004; 39: 630-634. [ Links ]
106. Meyer KA, Kushi LH, Jacobs Jr. DR, Folsom AR. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care 2001; 24: 1528-1535. [ Links ]
107. Van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002; 25: 417-424. [ Links ]
108. Ebbesson S. Fats and diabetes prevention in Inuits (abstract). Paper presented at the International Society for the Study of Fatty Acids and Lipids, Montreal, Canada, May 7-11, 2002. [ Links ]
109. Fritsche L, Weigert C, Häring HU, Lehmann R. How insulin receptor substrate proteins regulate the metabolic capacity of the liver - implications for health and disease. Curr Med Chem 2008; 15 (13): 1316-1329. [ Links ]
110. Karnieli E, Armoni M. Transcriptional Regulation of the insulin-responsive glucose transporter GLUT4 Gene: From Physiology to Pathology. Am J Physiol Endocrinol Metab 2008; 295 (1): E38-45. [ Links ]
111. Menzaghi C, Trischitta V, Doria A. Genetic influences of adiponectin on insulin resistance, type 2 diabetes, and cardiovascular disease. Diabetes 2007; 56: 1198-209. [ Links ]
112. Yong EL, Li J, Liu MH. Single gene contributions: genetic variants of peroxisome proliferators-activated receptor (isoforms alpha, beta/delta and gamma) and mechanisms of dyslipidemia. Curr Opin Lipidol 2008; 19: 106-112. [ Links ]
113. Rosenson RS. Effects of peroxisome proliferator-activated receptors on lipoprotein metabolism and glucose control in type 2 diabetes mellitus. Am J Cardiol 2007; 99 (4A): 96B- 104B. [ Links ]
114. Shalev A, Siegrist-Kaiser CA, Yen PM, Wahli W, Burger AG, Chin WW et al. The peroxisome proliferator-activated receptor is α phosphoprotein: regulation by insulin. Endocrinology 1996; 137 (10): 4499-4502. [ Links ]
115. Clarke SD, Thuillier P, Baillie RA, Sha X. Peroxisome proliferator-activated receptors: a family of lipid-activated transcription factors. Am J Clin Nutr 1999; 70: 566-571. [ Links ]
116. Berger J, Bailey P, Biswas C et al. Thiazolidinediones produce a conformational change in peroxisomal proliferator-activated receptor-γ: binding and activation correlate wit antidiabetic actions in db/db mice. Endocrinology 1996; 137: 4189-4195. [ Links ]
117. Lehmann J, Moore LB, Smith-Oliver TA, Wilkinson WO, Wilson TM; Kliewer SA. An antidiabetic thiazolidinedione is a hig affinity ligand for peroxisome proliferator-activated receptor γ (PPAR γ). J Biol Chem 1995; 270: 12953-12956. [ Links ]
118. Harris PKV. Kletzien RF. Localization of a pioglitazone response element in the adipocyte fatty acid-binding protein gene. Mol Pharmacol 1994; 45: 439-445. [ Links ]
119. Kletzien RF, Foellmi LA, Harris PKW, Wyse BM, Clarke SD. Adipocyte fatty acid binding protein (aFABP): regulation of gene expression in vivo and in vitro by an insulin-sensitizing agent. Mol Pharmacol 1992; 42: 558-562. [ Links ]
120. Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Eng J Med 1994; 331: 1188-1193. [ Links ]
121. Saha A, Kurowsky TG, Colca JR, Ruderman NB. Lipid abnormalities in tissues of the KKAy mouse mouse: effects of pioglitazone on malonyl-CoA and diacylglycerol. Am J Phisiol 1994; 267: E95-E101. [ Links ]
122. Hofmann C, Lorenz K, Colca J. Glucose transport deficiency in diabetic animals is corrected by treatment with oral antihyperglycemic agent pioglitazone. Endocrinology 1991; 129: 1915-1925. [ Links ]
123. Cardona F, Morcillo S, Gonzalo-Marín M, Garrido-Sánchez L, Macías-González M, Tinahones FJ. Pro 12Ala sequence variant of the PPARG gene is associated with postprandial hypertriglyceridemia in non-E3/E3 patients with the Metabolic Síndrome. Clin Chem 2006; 52: 1920-1925. [ Links ]
124. Macias-González M, Cardona F, Queipo-Ortuño M, Bernal R, Martín M, Tinahones FJ. PPARgamma mRNA expression is reduced in peripheral blood mononuclear cells after that overload in patients with metabolic syndrome. J Nutr 2008; 138: 903-907. [ Links ]
125. Lindi V, Schwab U, Louheranta A, Laakso M, Vessby B, Hermansen K et al. Impact of the Pro12Ala polymorphism of the PPAR- γ2 gene on serum triacylglycerol response to n-3 fatty acid suplementation. Mol Gen Metab 2003; 79: 52-60. [ Links ]
126. Perry JR, Frayling TM. New gene variants alter type 2 diabetes risk predominantly through reduced beta-cell function. Curr Opin Clin Nutr Metab Care 2008; 11: 371-377. [ Links ]
127. Simopoulos AP. Fatty acid composition of the skeletal muscle membrane phospholipids, insulin resistance and obesity. Nutr Today 1994; 2: 12-16. [ Links ]
128. Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med 2002; 19: 527-534. [ Links ]
129. Wallace TM, Levy JC, Matthews Dr. Use and abuse of HOMA modelling. Diabetes Care 2004; 27 (6): 1487-1495. [ Links ]
130. Morris AD, Ueda S, Petrie JR, Connell JM, Elliott HL, Donnelly R. The euglycaemic hyperinsulinaemic clamp: an evaluation of current methodology. Clin Exp Pharmacol Physiol (Australia) 1997; 24: 513-518. [ Links ]
131. Hirst S, Phillips D, Vines S, Clark P, Hales C. Reproducibility of the short insulin tolerance test. Diabet Med 1993; 10: 839-842. [ Links ]
132. Akinmokun A, Selby PL, Ramaiya K, Alberti KGMM. The short insulin tolerance test for determination of insulin sensitivity: a comparison with the euglycaemic clamp. Diabet Med 1992; 9: 432-437. [ Links ]
133. Bonora E, Targher G, Alberichie M, Bonadonna SG, Sagianni F, Zenere MB et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care 2000; 23: 57-63. [ Links ]
134. Emoto M, Nishizawa Y, Maekawa K, Hiura Y, Kanda H, Kawagishi T et al. Homeostasis model assessment as a clinical index of insulin resistance in type 2 diabetic patients treated with sulfonylureas. Diabetes Care 1999; 22: 818-822. [ Links ]
135. Lotz TF, Chase JG, McAuley KA, Shaw GM, Wong XW, Lin J, Lecompte A, Hann CE, Mann JI. Monte Carlo analysis of a new model-based method for insulin sensitivity testing. Comput Methods Programs Biomed 2008; 89 (3): 215-225. [ Links ]
136. Schoppen S, Sánchez-Muniz FJ, Pérez-Granados M, Gómez-Gerique JA, Sarriá B, Navas-Carretero S, Pilar Vaquero M. Does bicarbonated mineral water rich in sodium change insulin sensitivity of postmenopausal women? Nutr Hosp 2007; 22 (5): 538-544. [ Links ]
137. Hosker JP, Matthews DR, Rudenski AS, Burnett MA, Darling P, Bown EG et al. Continuous infusion of glucose with model assessment: measurement of insulin resistance and beta-cell function in man. Diabetología 1985; 28: 401-411. [ Links ]
138. Steil G, Murray J, Bergman R, Buchanan T. Repeatability of insulin sensitivity and glucose effectiveness from the minimal model. Implications for study design. Diabetes 1994; 43: 1365-1371. [ Links ]
139. Sheu WH, Hou JC, Jeng CY, Fuh MM. Reproducibility of the measurement of insulin sensitivity by the modified insulin suppression test. J Formos Med Assoc 1992; 91: 859-863. [ Links ]
140. Dorkhan M, Tripathy D, Malm G, Asgharian H, Tuomi T, Groop L. Independent measures of insulin secretion and insulin sensitivity during the same test: the glucagon-insulin tolerance test. J Intern Med 2008; 264 (1): 62-71. [ Links ]
141. Carrasco F. Síndrome metabólico: ¿más definiciones para una nueva enfermedad? Nutr Hosp 2006; 21 (2): 222-223. [ Links ]
142. Murphy KJ, Meyer BJ, Mori TA, Burke V, Mansour J, Patch CS et al. Impact of foods enriched with n-3 long-chain polyunsaturated fatty acids on erythrocyte n-3 levels and cardiovascular risk factors. Br J Nutr 2007; 97 (4): 749-757. [ Links ]
143. Von Schacky C. A review of omega-3 ethyl esters for cardiovascular prevention and treatment of increased blood triglyceride levels. Vasc Health Risk Manag 2006; 2 (3): 251-262. [ Links ]
144. Das UN. Essential Fatty acids - a review. Curr Pharm Biotechnol 2006; 7 (6): 467-482. [ Links ]
145. Das UN. Long-chain polyunsaturated fatty acids interact with nitric oxide, superoxide anion, and transforming growth factor-beta to prevent human essential hypertension. Eur J Clin Nut 2004; 58 (2): 195-203. [ Links ]
146. Mori TA, Beilin LJ. Long-chain omega 3 fatty acids, blood lipids and cardiovascular risk reduction. Curr Opin Lipidol 2001; 12 (1): 11-17. [ Links ]
147. Corridan B, Wilson A. Health effects of n-3 polyunsaturated fatty acids. In: Sadler MJ, Strain JJ, Caballero B, eds. Encyclopedia of Human Nutrition, Academic Press, vol. 2, pp. 757-69, London, 1998. [ Links ]
148. Martí-Cid R, Bocio A, Llobet JM, Domingo JL. Intake of chemical contaminants through fish and seafood consumption by children of Catalonia, Spain: health risks. Food Chem Toxicol 2007; 45: 1968-1974. [ Links ]
![]() Correspondence:
Correspondence:
M. P. Vaquero.
Department of Metabolism and Nutrition.
Instituto del Frío. ICTAN (CSIC).
C/ José Antonio Novais, 10.
28040 CSIC. Madrid. Spain.
E-mail: mpvaquero@if.csic.es
Recibido: 26-IX-2008.
Aceptado: 25-XI-2008.