Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.24 no.4 Madrid jul./ago. 2009
Viscosity and flow-rate of three high-energy, high-fibre enteral nutrition formulas
Viscosidad y flujo de caída libre de tres fórmulas de nutrición enteral ricas en energía y fibra
P. Casas-Augustench1 and J. Salas-Salvadó1,2
1Human Nutrition Unit. Hospital Universitari Sant Joan de Reus. IISPV. Rovira i Virgili University. Reus. Spain.
2CIBER Fisiopatología de la Obesidad y Nutrición (CB06/03). Instituto de Salud Carlos III (ISCIII). Madrid. Spain.
We sincerely thank Nestlé Healthcare Nutrition S.A., for their support in carrying out this study
ABSTRACT
Introduction: There have been few studies evaluating how the viscosity of the enteral nutrition formulas determine the time of nutritional administration by gravity and whether viscosity causes tubes to become obstructed.
Objective: To assess how long it takes for three polymeric, hypercaloric and fibre-rich enteral nutrition formulas marketed in Europe to pass through different nasointestinal tubes by gravity and whether these formulas obstruct the tubes.
Methods: We evaluated the in vitro viscosity of the three formulas using a rotational viscometer and by calculating how long these formulas took to pass by free fall through the equipment and different calibre tubes. We also assessed the possible obstruction of the tubes or the equipment after the three formulas had been administered, simulating the administration conditions in clinical practice (1,500 ml over 24 h).
Results: The administration time by gravity of 500 ml of each of the formulas studied was closely related to the viscosity determined in vitro of each of the formulas used. The larger the internal diameter of the tube, the shorter the emptying time by gravity or free fall. The possibility of tube obstruction was higher in the case of the two more viscous formulas.
Conclusions: The viscosity of the enteral nutrition formulas should be included in the labelling of the product. This information would assist the clinician to make decisions about the kind of formula to be used with different types and calibres of tube.
Key words: Enteral nutrition. Nasoenteric tube. Viscosity. Time of administration.
RESUMEN
Introducción: Existen escasos estudios que evalúen el efecto de la viscosidad de las fórmulas de nutrición enteral sobre el tiempo de administración de la nutrición por caída libre y la posibilidad de obturación de la sonda utilizada.
Objetivos: Evaluar la presencia de obturación y tiempo de paso de tres fórmulas poliméricas hipercalóricas y ricas en fibra de nutrición enteral a través de diferentes sondas nasointestinales por gravedad.
Métodos: Se evaluó la viscosidad in vitro de tres fórmulas mediante un viscosímetro rotacional y el tiempo por caída libre a través de un equipo y sondas de diferente calibre de tres fórmulas ricas en fibra comercializadas en Europa. También se evaluó la presencia de obturaciones de la sonda o el equipo tras la administración de las tres fórmulas simulando las condiciones de administración en la práctica clínica (paso de 1.500 ml durante 24 h).
Resultados: El tiempo de administración por gravedad de 500 ml de cada una de las fórmulas se relacionó estrechamente con la viscosidad determinada in vitro de cada una de las fórmulas utilizadas. A mayor diámetro interno de la sonda, menor fue el tiempo de vaciado por caída libre o gravedad. La posibilidad de obturación de la sonda fue mayor en el caso de las dos fórmulas más viscosas.
Conclusión: La viscosidad de las fórmulas de nutrición enteral debería figurar en el etiquetaje del producto. Esta información ayudaría al clínico a tomar decisiones sobre el tipo de fórmula a emplear en función del tipo y calibre de sonda a utilizar.
Palabras clave: Nutrición enteral. Sonda nasogástrica. Viscosidad. Tiempo de administración.
Introduction
The development and deployment of artificial nutrition techniques has brought about the possibility of increasing life expectancy in different situations and diseases. The use of enteral nutrition has grown in recent years.1-3 This is partly because the parenteral support that often was used in some situations has not shown to be advantageous compared to the enteral feeding technique.
Enteral nutrition through nasogastric or nasointestinal tubes is the most commonly used technique in artificial nutritional support both in hospitals and outpatients. The method of tube administration most frequently used in chronic patients is intermittent or continuous by gravity. However, in acute care hospitals, continuous administration through a pump is also widely used in different situations requiring continuous input of small quantities of nutrients over time. This promotes the proper use of nutrients and reduces the risks associated with the slowdown of the normal gastrointestinal transit.
Tube obstruction is a relatively common complication that can be seen both in the hospital and at home. Obstruction stops the administration of nutrients and sometimes medication and this has consequences for the individual. In addition, the obstructed tube often has to be replaced by another tube, which means added inconvenience and risks for the patient.4,5
On many occasions, the passage of nutrients through the tube also slows down because of the viscosity of the formula administered and/or the small size of the tube used.6,7 This may mean the nutrients are inadequately administered and thus can lead to negative consequences and inconvenience for both caregiver health personnel and family members or patients themselves.
It has been previously seen that using highly viscous hypercaloric formulas may lead to an increased risk of obstructing tubes as well as a delay in administration. However, there are few studies that evaluate the effect of the type of formula and tube on the time required to administer enteral nutrition by gravity and the possibility of tube obstruction1.
Therefore, the purpose of this study is to assess three high-energy, high-fibre enteral nutrition formulas for their viscosity, their falling time using gravity and their likelihood of causing obstruction in nasoenteric tubes of different calibres.
Material and methods
This study was divided into three phases: a) in vitro viscosity determination of three hypercaloric and rich in fibre enteral nutrition formulas; b) comparison of the time needed to pass 500 ml of the three enteral nutrition formulas using different calibre nasoenteric tubes by free fall; and c) assessment of the possibility of tube obstruction after administration by gravity of 1,500 ml of the three enteral nutrition formulas for 24 hours.
Enteral nutrition formulas and tubes used
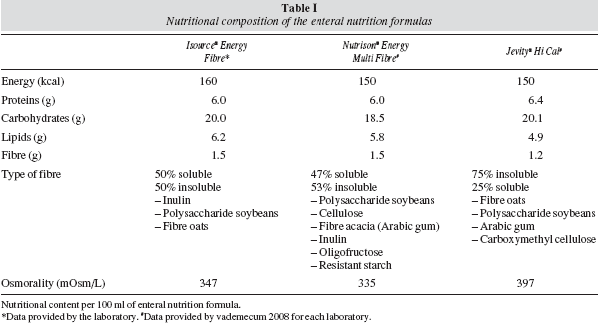
We compared three hypercaloric enteral nutrition formulas (between 1.5 and 1.6 kcal/ml) enriched in fibre and marketed in Europe; table I shows their composition. Two of the three formulas with similar caloric content had the same energy density (Nutrison® Energy Multi Fibre and Jevity® Hi Cal). Both the quantity and the type of fibre (soluble/insoluble relationship) were very similar in the case of the formula Isosource® Energy Fibre and Nutrison® Energy Multi Fibre.
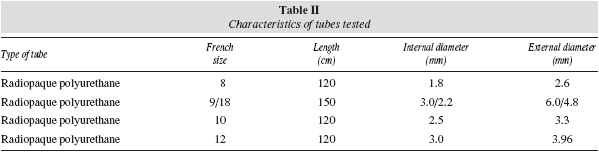
Three polyurethane nasogastric tubes with different calibre (Compat® 8, 10 and 12 French, Nestle Healthcare Nutrition, SA) and one nasojejunal tube (Compat® Stay-Put 9/18 French, Nestle Healthcare Nutrition, SA) were used for evaluating the infusion time and the possibility of obstruction. Table II shows the characteristics of each of the tubes used.
Viscosity assessment
The viscosity of the enteral nutrition formulas was determined in triplicate in vitro through a rotational viscometer Brookfield (Brookfield Engineering, Stoughton, MA) at 25º C. The average of the three determinations was the accepted value.
Fluidity determination by free fall
The fluidity of the formulas was estimated by observing the time of administration by free fall of each of the formulas. After the formula had been shaken (500 ml) for 30 seconds, it was hung so that the distal end of the tube was located 10 cm from the ground. The administration speed of the line was graduated at the highest possible speed (Roller totally opened). The administration of the formula was timed from the beginning to finish. The presence or absence of obstructions was recorded and, if appropriate, the time at which the stoppage occurred.
The experiment was conducted at 25º C and it was repeated three times for each formula using each of the nasogastric tubes described. The average of the three determinations was the accepted value.
Determining possible tube obstruction
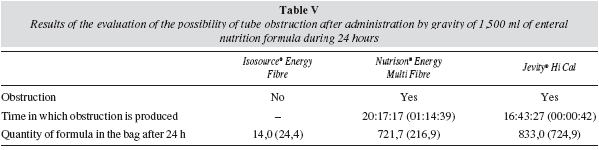
Possible tube obstruction was determined after the administration of 1,500 ml of the three enteral nutrition formulas by gravity. Following agitation of the formula for 30 seconds, it was transferred to a bag of enteral nutrition, which was hung so that the distal end of the tube was located 10 cm from the ground. The infusion was graduated at a velocity of 1-drop/3 seconds (1 drop is equivalent to 20 μL). The administration of the formula was timed from the beginning to finish. The presence or absence of obstructions was recorded and, if appropriate, the time at which the stoppage occurred. The amount of formula that remained in the bag after the end of the administration was also weighed. With this test, we attempted to simulate as closely as possible the normal clinical conditions of tube administered enteral nutrition (administration of 1,500 ml in 24 hours).
The experiment was conducted at room temperature of 25° C and was repeated three times for each formula using the tube (9/18FR). For this experiment, nasojejunal tube (9/18) was used since this is the tube in which obstructions are most frequently observed in clinical practice, particularly with products rich in fibre.
Statistical methods
Results are shown as means and standard deviations.
Results
The first phase of the study showed that despite similar fibre and energy density input, the viscosities of the three formulas studied were different. The formula with less viscosity in vitro was Isosource® Energy Fibre, followed by Nutrison® Energy Multi Fibre and Jevity® Hi Cal, which proved to be the most viscous (table III).
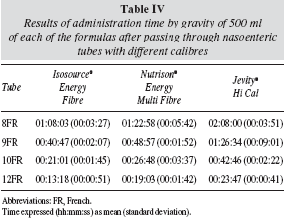
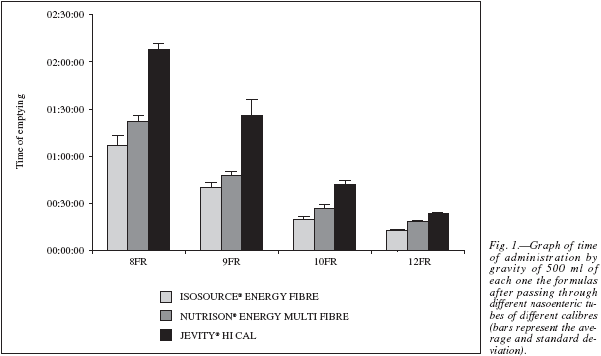
In the second phase of the study, it was observed that the administration time of 500 ml of each one of the formulas was closely related to the viscosity determined in vitro of each of the formulas used, with the most viscous formula taking the longest to passthrough different calibre nasoenteric tubes (table IV, fig. 1). Moreover, the larger the internal diameter of thetube, the shorter the emptying time by gravity or free fall. There was no obstruction of the tube during theadministration of 500 ml of the formulas by free fall(Roller fully opened) through the different calibre nasogastric tubes used in this experiment.
In the third phase of the study, which reproduces the clinical conditions for the routine use of formulas for enteral nutrition, an obstruction was observed after the free fall administration of Nutrison® Energy Multi Fibre and Jevity® Hi Cal formulas through a 9/18 FR tube of 1,500 ml/24 h. However, no obstruction was observed with Isosource® Energy Fibre formula when this volume was administered with the same tube and conditions, so the residual volume in the bag of enteral nutrition was practically nil at the end of the infusion. As can be seen in table 5, the amount of formula that remained in the bag after the obstruction was higher in the case of the Jevity® Hi Cal formula compared to the Nutrison® Energy Multi Fibre formula.
Discussion
During recent years, the pharmaceutical market has developed a large number of formulas for enteral nutrition and improved techniques and materials related to its administration. This development has helped to improve the nutritional status of patients who need to be fed artificially and thus, promote their quality of life both at home and hospital.
Despite the significant growth in number of formulas and materials, there are still several issues to consider when it comes to managing enteral diets to avoid possible complications and the possible obstructions of the tubes.
This work has allowed the study in vitro of the viscosity of three enteral nutrition formulas available in the European market affects the time of nutritional administration by free fall. The viscosity determined in each of the formulas used conditioned the fall times through tubes with different calibres. Thus, the formulas that were more viscous took more time to flow gravitationally.
The recommended way of administering to avoid the dumping effect or diarrhea is 240 to 480 ml of diet over a period of 20-40 minutes from 4 to 6 times a day1. Following the results shown in Table 4, this criterion is satisfied only by the following relationships between tubes and formulas: a) tube 9FR with Isosource® Energy Fibre formula, b) tube 10FR with Isosource® Energy Fibre or Nutrison® Energy Multi Fibre formulas, and c) tube 12FR with Isosource® Energy Fibre, Nutrison ®Energy Multi Fibre or Jevity® Hi Cal formulas. Therefore, it is desirable not to use other combinations to avoid complications such as a delay in the diet administration.
The three formulas studied are similar in nutritional composition, being rich in fibre and energy. These properties mean these formulas have greater viscosity8 and special care is needed when choosing the most appropriate type of tube to avoid possible obstructions or other complications. Although Isosource® Energy Fibre formula is the most caloric and one of the richest in fibre, it was the one that took the least time to administer by gravity through the various nasoenteric tubes with different calibre used. Nutrison® Energy Multi Fibre formula has the same fibre content but is more viscous than Isosource® Energy Fibre formula. The time taken by Nutrison® Energy Multi Fibre formula through the different tubes was higher than for Isosource® Energy Fibre formula. This could be explained by differences in the viscosity values of these two products or differences in the source of fibre used (in the case of Jevity® Hi Cal, 75% is insoluble while in the other two formulas this is approximately 50%). Jevity® Hi Cal formula is the most viscous and quickest to pass through the different tubes used, despite having a slightly lower content of fibre/100 ml compared to the other formulas studied.
The degree of viscosity of a formula depends crucially on the density of nutrients incorporated and the type and quantity of stabilizers used as well as the presence of networks of dispersed particles (especially proteins and polysaccharides) and emulsifiers used to avoid coalescence, sedimentation and phase separation of the formula.9,10 Some circumstances of the development process are also essential in viscosity and degree of dispersion, pH, homogenization and temperature during the processing, since they alter the possible interactions between macromolecules present in solution.9,10 This could explain why the three formulas similar in energy density and amount of fibre (two of them nearly identical) have different viscosities, as we observed in our study.
Dietary fibre is essential in maintaining proper bowel function. Currently it is believed that fibre must be present in preparations for enteral nutrition to manage multiple situations, but the reality is that routine enteral nutrition often indicated by clinicians does not contain fibre.7 This is because the formulas rich in fibre can generate problems of: a) administration such as slowing the infusion speed or obstructing the tube if the calibre that is very fine, or b) gastrointestinal intolerance. However, in this experiment there was no tube obstruction during the administration of 500 ml of formula through the various tubes used, even using formulas which were highly viscous and rich in fibre. Nevertheless, this does not mean that these formulas could not produce any obstruction in vivo, since there are other factors that cannot be taken into account in an in vitro study such as the type of formula used, contact between the formula and the gastric pH, and the formula's interaction with the medications administered by the same tube. In addition, in our experiment the roller was totally opened, which is not habitual in vivo administration conditions.
However, when considering the presence of obstructions in tube 9/18 FR after administration of 1,500 ml/24h of the three enteral nutrition formulas by gravity, obstructions were observed with Nutrison® Energy Multi Fibre and Jevity® Hi Cal formulas. The same phenomenon was not observed for Isosource® Energy Fibre formula. Differences in the viscosity and content/type of fibre of these formulas could explain these divergences.
In conclusion, some of the enteral nutrition formulas marketed in Europe exceeded the recommended time for administration by gravity. To avoid delays in the administration of the formulas or other possible complications, the viscosity of the enteral nutrition formulas should be included in the labelling of the product. This information would assist the clinician in making decisions about the kind of formula to be used for different types and calibres of tube. In addition, the professional who uses these products should be informed of the type of tubes that should be used according to the viscosity of the product.
Acknowledgment
We sincerely thank Nestlé Healthcare Nutrition S.A., for their support in carrying out this study. PCC contributed to the design of the study, acquisition and analysis of data, interpretation of results and drafting the manuscript. JSS contributed to the design and coordination of the study, interpretation of results and drafting of the manuscript with intellectual and scientific input. All authors gave their final approval of the submitted manuscript.
References
1. Montejo O, Alba G, Cardona D, Estelrich J, Mangues MA. Relación entre la viscosidad de las dietas enterales y las complicaciones mecánicas en su administración según el diámetro de la sonda nasogástrica. Nutr Hosp 2001; 16 (2): 41-45. [ Links ]
2. Álvarez Hernández J, Peláez Torres N, Muñoz Jiménez A. Clinical use of enteral nutrition. Nutr Hosp 2006; 21 (Supl. 2): 85-97, 87-99. [ Links ]
3. Cuerda C, Chicharro ML, Frías L, García Luna PP, Cardona D, Camarero E, Penacho MA, Calañas A, Parés RM, Martínez Olmos MA, Zapata A, Rabassa Soler A, Gómez Candela C, Pérez de la Cruz A, Lecha M, Luis D, Luengo LM, Wanden-Berghe C, Laborda L, Matía P, Cantón A, Martí E, Irles JA; grupo NADYA-SENPE. Registry of home-based enteral nutrition in Spain for the year 2006 (NADYA-SENPE Group). Nutr Hosp 2008; 23 (2): 95-9. [ Links ]
4. Grant JP. Técnicas de acceso no invasivas para nutrición enteral. En: A. Esteban, S. Ruiz Santana, T. Grau. (ed): Alimentación enteral en el paciente grave. 2ª Ed. Barcelona, Springer-Verlag Ibérica S.A, 1994, pp. 99-118. [ Links ]
5. Kripke SA, Rombeau JL. Técnicas invasivas para nutrición enteral. En: A. Esteban, S. Ruiz Santana, T. Grau. (ed): Alimentación enteral en el paciente grave. 2ª Ed. Barcelona, Springer-Verlag Ibérica S.A, 1994, pp. 109-118. [ Links ]
6. Serrano L, Palma F, Carrasco F, Guinda A. The relation between the viscosity of enteral nutrition products and delays or interruptions in the infusion rate selected. Nutr Hosp 1994; 9(4): 257-61. [ Links ]
7. Gómez Candela C, De Cos Blanco AI, Iglesias Rosado C. Fibre and enteral nutrition. Nutr Hosp 2002; 17 (Supl. 2): 30-40. [ Links ]
8. Gottschlich M, Politzer E y Hutchins AM: Defined formula diets. En: Romboau J, Rolandelli R (ed.): Clinical nutrition. Enteral and tube feeding 3rd ed. Philadelphia: WB Sounders Company, 1997: 226. [ Links ]
9. Fennema OR. Química de los alimentos. Ed. Acribia: Zaragoza, 1993. [ Links ]
10. Belitz & Grosch. Food Chemistry, 2nd ed. Springer-Verlag: Berlin, 1999. [ Links ]
![]() Correspondence:
Correspondence:
Jordi Salas-Salvadó.
Human Nutrition Unit.
Department of Biochemistry and Biotechnology.
Faculty of Medicine and Health Sciences.
University Rovira i Virgili.
C/ Sant Llorenç, 21.
43201 Reus.
E-mail: jordi.salas@urv.cat
Recibido: 11-VIII-2008.
Aceptado: 2-IX-2008.