My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.25 n.2 Madrid Mar./Apr. 2010
Influence of low-protein dietetic foods consumption on quality of life and levels of B vitamins and homocysteine in patients with chronic renal failure
Influencia del consumo de alimentos dietéticos bajos en proteína sobre la calidad de vida y los niveles de vitaminas B y homocisteína en pacientes con insuficiencia renal crónica
C. Sánchez1, P. Aranda1, E. Planells, P. Galindo2, A. Pérez de la Cruz3, M. Larrubia4 and J. Llopis1
1Institute of Nutrition and Food Technology and Department of Physiology. Cartuja Campus. University of Granada. Granada. Spain.
2Nephrology Dept. Virgen de las Nieves University Hospital. Granada. Spain.
3Nutrition and Dietetics Dept. Virgen de las Nieves University Hospital. Granada. Spain.
4Dietetic Foods. Sanavi. Lachar. Granada. Spain.
This research was supported by Plan Nacional I+D project 1FD 1997-0642. All authors have contributed to the experimental work, and to the development and final discussion of the manuscript. We thank Sanavi S.A. for supplying the dietetic products.
ABSTRACT
Objective: The aim of the study was to determine whether the consumption of low protein dietetic foods® improved the quality of life and nutritional status for vitamins B and homocysteine in patients with chronic renal failure.
Methodology: This nutritional-intervention involved 28 men and 21 women, divided into two groups. The control-group consumed a low-protein diet prescribed, and the experimental-group consumed a diet in which some commonly used foods were replaced by low-protein dietetic foods. The study lasted 6 months. Food consumption was assessed by 24-h recall. Vitamin B6 as αEAST was measured in blood. Creatinine, urea, vitamin B12, folate and homocysteine were measured in plasma. The impact on the patients' quality of life from consuming the dietetic foods was assessed via the SF-36 questionnaire.
Results: After 6 months, the protein intake among the experimental-group had decreased by 40%, and the urea/creatinine ratio and αEAST activity were also lower. The results of the SF-36 questionnaire show that the patients in the experimental-group obtained higher scores in the categories of general health and physical status.
Conclusions: The dietetic foods were very well accepted by all patients and their use allowed a better control of the protein intake, improved B6 status and a better quality of life.
Key words: Chronic renal failure. Vitamins B. Homocysteine. Dietetic foods. Quality of life.
RESUMEN
Objetivo: Se estudió si el consumo de productos dietéticos bajos en proteína® mejora la calidad de vida y el estado nutricional en vitaminas B y homocisteína en pacientes con insuficiencia renal crónica (IRC).
Material y métodos: La intervención nutricional se llevó a cabo durante 6 meses en un grupo de pacientes con IRC (28 hombres y 21 mujeres) divididos en dos grupos. El grupo control consumió la dieta prescrita para la IRC. El grupo experimental consumió una dieta en donde parte de los alimentos fueron sustituidos por los productos dietéticos bajos en proteína. El consumo de alimentos fue analizado mediante recordatorio de 24 horas. El estatus en Vitamina B6 se determinó como αEAST en muestras de sangre. En plasma se analizaron los niveles de creatinina, urea, Vitamina B12, folato y homocisteína. El impacto del consumo de los productos dietéticos sobre la calidad de vida de los pacientes se analizó mediante el cuestionario de salud SF-36.
Resultados: Tras 6 meses de intervención nutricional, la ingesta proteica en el grupo experimental descendió un 40%. También se redujo la relación urea/creatinina y la actividad αEAST. Los resultados del cuestionario de salud SF-36 mostraron que los pacientes del grupo experimental obtuvieron mejores puntuaciones en las categorías de salud general y estado físico.
Conclusiones: Los productos dietéticos bajos en proteína fueron muy bien aceptados por los pacientes y su uso permitió un mejor control de la ingesta proteica, mejorando el estado nutricional del paciente en B6 y sucalidad de vida.
Palabras clave: Insuficiencia renal crónica. Vitaminas B. Homocisteína. Estado nutricional. Alimentos dietéticos. Calidad de vida.
Introduction
Chronic renal failure (CRF) alters the metabolism of and nutritional requirements for a number of vitamins, and can lead to either deficiency or raised levels of these nutrients. Among the causes of these alterations are reduced food intake, the low vitamin content of some low-protein diets, increased endogenous breakdown or clearance of vitamins from the blood, and interference by certain drugs.
Although low-protein diets have often been recommended as a means of slowing the progression of CRF1,2 adherence to these diets is poor because of the lack of variety in menus and the limits on the consumption of many foods.3 Moreover, reduced food intake in these patients often worsens the situation and increases the chances that the intake of micronutrients such as folate and vitamins B6 and B12, abundant in protein-rich foods, will also be inadequate. These vitamins are known to be involved in the metabolism of homocysteine, and deficient levels favour hyperhomocysteinemia. This cardiovascular risk factor is particularly worrisome in patients with CRF. In patients with uraemia the mortality rate attributable to cardiovascular disease is 30% higher than in the general population, and hyperhomocysteinemia is the most prevalent risk factor.4,5.
This study was designed to discover whether the consumption of manufactured low-protein dietetic foods by patients with CRF enables them to better adjust their protein intake and to maintain their nutritional status for vitamins B6, B12and folate, and to decrease hyperhomocysteinemia. Moreover, it is important to determine whether the use of these dietetic products might facilitate the manufacture and variety of diets, and thus improve users' quality of life.
Methods
Patients
The participants in this clinical-nutritional intervention were patients with CRF on predialysis. The following criteria were established for inclusion: serum creatinine concentration > 2.5 mg/dL, plasma creatinine clearance between 10 and 45 mL/min, stable clinical condition (stable blood pressure, no special diet, no digestive system or systemic disease, neoplasias, or treatment with corticosteroids or immunosuppressors), corrected metabolic acidosis and lipid alterations, age between 18 and 70 years, and knowing how to read and write. The study was authorized by the Ethics Committee of the Hospital Universitario Virgen de las Nieves (Granada, Spain). All patients provided their consent by signing an Informed Consent form.
The sample was consecutive and nonprobabilistic, since we included all patients who met the inclusion criteria and were seen at the nephrology outpatient clinic of the Hospital Universitario Virgen de las Nieves (Granada, Spain) between November 1999 and June 2006.
The sample of patients initially invited to participate consisted of 64 men and women aged 18 to 70 years. The final sample consisted of 49 persons (28 men, 21 women) with a mean age of 55 (SD 16) years. The final participation rate was 76.6%, and the reasons for dropout, or withdrawal by the investigators were scheduled dialysis (26.7%), nonadherence to the diet(60.0%) or death (13.3%).
The participants were divided randomly into two groups. The 25 control participants (C) remained on the low-protein diet recommended by the hospital. This diet was based on a weekly low-sodium menu that supplied a mean of 46.3 g protein/day, 54.6 g fat/day and 240 g carbohydrates/day. The 24 participants in the experimental group (E) were instructed by a trained dietician to consume a conventional low-protein diet, but with some of the foods commonly used being replaced by dietetic foods low in protein, amino acids, sodium, potassium and phosphorus, and enriched with certain minerals and vitamins (Harifen®, SanaviSA, Granada, Spain). Harifen manufactures a wide range of dietetic foods especially suitable for the treatment of diseases related to protein metabolism, such as chronic renal disease and metabolic disorders. Further information about the use, composition and manufacture of these foods can be obtained from: http://www.sanavi.com/ing/harifen/harifen.html.
The following dietetic foods were supplied to the patients during the study: prebaked bread, milk substitute, Italian pastas, rice substitute, toasted bread, baking and pastry mixture, and various types of biscuits.
The participants were advised by a dietician on how to prepare their meals in accordance with nutritional recommendations for CRF,6 replacing the foods normally consumed by the special, low-protein dietetic ones supplied. These training sessions were personalized and lasted for 1 week.
The recommended diet contained 0.6 g protein (50% high biological value)/kg bodyweight per day7,8 and 35 kcal/kg bodyweight per day9 and was low in sodium, potassium, phosphates, saturated fat and refined sugar.
Participants with obesity (50%) and those older than 60 years (47.3%) were advised to consume a diet that provided 30 kcal/kg ideal weight per day. To adjust the energy content of the low-protein diet, obesity was considered to exist when the participant weighed more than 125% of his or her ideal weight.10.
On day 0 of the study, all participants received a physical examination, and clinical and nutritional data were recorded. During the 6-month experimental phase, participants in group E consumed the low-protein diets they designed themselves during the initial dietary intervention, while participants in group C continued to consume the low-protein diet recommended by the hospital.
Pharmacological treatment was similar for all participants, and was adjusted depending on individual clinical status. Medications used by participants in this study included calcium-chelated phosphate, calcitriol, oral sodium bicarbonate, ferrous sulphate, antihypertensives (mainly angiotensin-converting enzyme inhibitors), furosemide and subcutaneous erythropoietin (EPO).
At the start of the study and after 6 months, blood samples were taken for biochemical analysis and food consumption was assessed by 24-h recall on 3 different days (including a weekend or holiday).11 Food consumption data were obtained by a dietician with the aid of an open questionnaire and photographs as a reference for portion size. The pictures showed fresh foods or foods prepared according to usual recipes for dishes that are widely consumed in the area. Food intakes were converted into energy and nutrients with the help of the Spanish Food Composition Table.12 The composition database was used with AYS44 Diet Analysis software from ASDE, SA (Valencia, Spain).
In order to determine, from the patient's viewpoint, the impact on quality of life from consuming these lowprotein dietetic foods, all patients were asked to fill in the SF-36 health status questionnaire13 at the start and end of the study. This questionnaire consisted of 36 items divided into 8 categories, addressing the patient's perception of his/her health status in general, social function, physical status, limitations caused by physical and/or emotional problems, mental health, vitality and pain. A score for each category was calculated, ranging from 0 to 100. This questionnaire enabled us to detect both positive and negative states of health, and to categorise mental and physical health status, as well as any changes in health status occurring during the treatment period.
Analytical methods
Blood was collected in the morning after the participants had abstained from eating and drinking overnight. Creatinine, urea, uric acid, albumin and total protein concentrations were measured with enzymatic colorimetric tests in a Hitachi Modular P autoanalyzer (Roche Diagnostics, Grenzach, Germany).
Part of the blood (6 mL) was collected in tubes that contained 1 mL ACD-stabilizer (Venoject, Terumo Corporation, Leuven, Belgium). The samples were centrifuged at 3000 × g for 15 min at 20 ºC to separate plasma, and erythrocytes were washed with isotonic saline and stored at -80 ºC until analysis.
To assess B6 nutritional status, we used erythrocyte aspartate aminotransferase activity (EAST) with and without added pyridoxal phosphate (PLP) (Sigma, St Louis, MO). The activation coefficient (a) for erythrocyte aspartate aminotransferase (aEAST) was taken as the ratio of activity with added PLP to activity without PLP.14 The cutoff points for aEAST were > 1.85 for high risk, 1.85-1.70 for low (moderate) risk, and < 1.70 for acceptable (low) risk.14,15.
Vitamin B12 and folate were measured with an electrochemical luminescence immunoassay (ECLIA, Elecsys 2010 and Modular Analytics E 170, Roche Diagnostics, Germany). The reference value for vitamin B12 was 150 μg/L and the reference value for folic acid was 3 pg/L.15
Plasma homocysteine concentration was measured with a fluorescence polarization immunoassay (FPIA) (IM® Abbott Laboratories, Abbott Park, IL, USA).
Statistical analysis
All variables and indexes were analyzed with descriptive statistics to report mean values and standard deviations. When the data were distributed normally according to the Kolmogorov-Smirnov test, we used parametric tests, i.e., Student's t test for independent or related samples. For variables that required nonparametric testing we used the Wilcoxon test for related samples and the Mann-Whitney test for unrelated samples.
Linear regression analysis was used to find bivariate correlations; Pearson's correlation coefficient was calculated for 95% confidence levels. Multiple logistic regression analysis was used to estimate the degree of association between intake or plasma values (dependent variable) and gender, age, group (control and experimental) and experimental period (day 0 and month 6). The model was adjusted for all variables. All analyses were performed with version 14.0 of the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL). Differences were considered significant at the 5% probability level.
Results
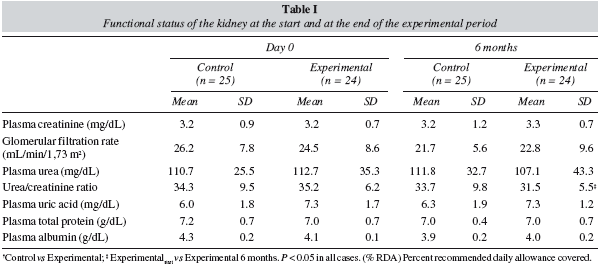
Table I gives the results for the biochemical indicators of renal function. Among the control participants, the glomerular filtration rate (GFR) decreased by 17.2% (4.5 mL/min), whereas for the participants in the experimental group who received nutritional education and lowered their protein intake, the decrease was only 6.9% (1.7 mL/min). In the experimental group, the urea/creatinine ratio was significantly reduced after six months, with respect to the initial values recorded, while no changes were observed among the control group.
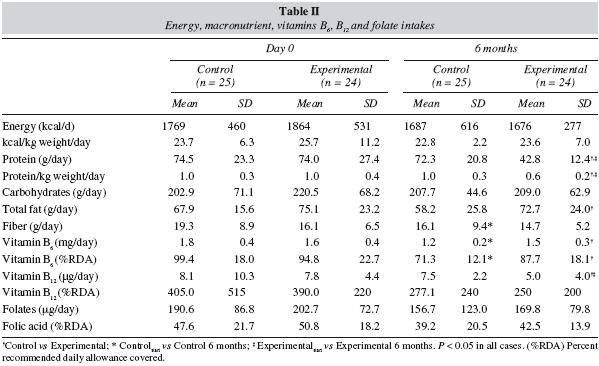
Table II summarizes the data for intakes of energy, macronutrients and vitamins, and the percentages of the RDA covered at the start of the experimental period and after 6 months. Energy intakes were below the RDA9 and tended to decrease with time in both the control and experimental groups. Protein intake in the experimental group (E) decreased significantly and attained the recommended value of 0.6 g protein/kg weight/day.8 In the former, protein intake decreased by 40% (from 1.0 to 0.6 g protein/kg weight/day). More over, among the experimental group, there was a significant decrease in total fat.
On the other hand, by the end of the study, the intake of vitamin B6 among the experimental group was significantly higher than among the control group. The intake of vitamin B12 decreased among the experimental group, while no significant changes were recorded for the intake of folates.
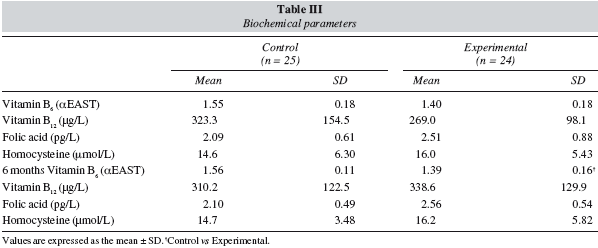
Table III shows the blood and biochemical parameters for vitamins and homocysteine. After 6 months, EAST activity among the experimental group was lower (P < 0.05) than in the control group.
Values for vitamin B12 were below the reference value (150 μg/L) in two control patients, both of whom continued to present similarly low values at the end ofthe study period.
The results for circulating homocysteine levels indicated moderate hyperhomocysteinemia at the start of the study, which was maintained in both the control and experimental groups during the study period.
Linear regression analysis shows that vitamin B6 intake correlated with energy intake (r = 0.49; P < 0.01), protein intake (r = 0.50; P < 0.001) and vitamin B12 intake (r = 0.60, P < 0.001). Vitamin B12 intake correlated with protein intake (r = 0.34; P < 0.05). Folate intake correlated with energy intake (r = 0.44; P < 0.01), protein intake (r = 0.41; P < 0.01) and vitamin B6 intake (r = 0.34; P < 0.05).
Logistic regression analysis did not reveal any significant associations between intake or plasma values (dependent variable) and gender, age, group (control or experimental) or experimental period (day 0 or at 6 months).
The results obtained from the SF-36 health status questionnaire suggest that by the end of the study period, the patients who consumed the low-protein dietetic foods presented higher scores in the categories of general health (Mean, SD) [68, 1.8 (control) vs 72, 1.2 (experimental), P < 0.05] and physical status [46, 1.2 (control) vs 56, 2.0 (experimental), P < 0.05].
Discussion
Analysis of the biochemical parameters commonly used in clinical practice to study renal function (Table 1) showed that in the experimental group, a lower protein intake led to a smaller decrease in GFR, as has been reported in other studies.16 The urea/creatinine ratio in both groups in the present study indicated a slight excess in protein levels (recommended values between 20 and 30 mg/dL). Nevertheless, this was far below the cutoff value for excess protein intake (> 40 mg/dL).17 The significant decrease in the urea/creatinine ratio observed among the experimental group resulted from the slight fall in circulating levels of urea, a consequence of the consumption of low-protein dietetic products (tables I and II).
Participants in the experimental group received nutritional information to enable them to design a lowprotein diet that covered their needs18 and replace foods commonly consumed with dietetic substitutes. These were very well accepted by all patients.
The results of the food consumption analysis show that energy intake failed to cover 100% of the RDA at the start of the study or after 6 months (table II). This situation might reflect the reduced food intake often seen in patients with CRF.19
At the beginning of the study, protein intakes were similar in the control and experimental groups, at approximately 170% of the recommended value, reflecting the fact that high protein intakes are common among the adult population in southern Spain20. The nutritional training programme appeared to be an important factor in reducing protein intake, since our participants attained the prescribed value of 0.6 g protein/kg weight/day (table II).
Several studies have reported that vitamin B6 status worsens in CRF.21 Low-protein diets can lead to deficient intakes of vitamin B6, supplied mostly by meat and fish among our population22. The decreased vitamin intake may explain why intakes in both groups were close to the RDA at the beginning of the study but were lower after 6 months. This change paralleled the above-described pattern of protein intake. However, although protein intake fell much more sharply among the experimental group than among the control group, the fall in pyroxidine intake was lower among the experimental group (table II). This is because the dietetic products supplied were enriched in this vitamin. In this respect, the mean values measured in our participants were slightly lower than those found by Kopple et al.23 in adults with advanced CRF.
The greater consumption of vitamin B6 among the experimental group meant that the average activity of EAST decreased among this group, which indicates an improvement in the status of this vitamin among the patients.
The low-protein diet consumed by the experimental group led to a lower intake of vitamin B12 because in southern Spain the main sources of this nutrient are meat and fish22. The linear correlation between vitamin B12 and protein intake (see Results) supports this hypothesis. Nevertheless, mean intakes were substantially higher than the RDA (table II) in all cases.
Mean plasma concentrations of vitamin B12 are usually within the normal range in patients with CRF,24 and deficiencies are rarely encountered because of the low requirements for this vitamin. However, the plasma mean values were clearly lower than those observed in earlier studies in the healthy adult population residing in the same geographical area.22.
The relationship between CRF and the RDA for folate is a controversial question. Our results showed that folate intake was low in both groups at the beginning of the study and after 6 months, and that most patients failed to cover 50% of the RDA. This finding appeared to be related to low energy intakes, a hypothesis supported by the linear correlation between folate and energy intake (see Results). Although folate intakes were far below the RDA for the healthy adult population, intakes approached the estimated content (260 μg/day) of different low-protein diets (40 g/day) prescribed to patients with CRF.1
A high incidence of folate deficiency has been reported in patients with CRF who are not receiving dialysis. It has also been found, however, that in patients with moderate CRF, serum concentrations of folate are normal. 1 Mean values for folate at the start of the present study and after 6 months were slightly below the reference value (3 pg/L). Most patients (79.6%) had plasma concentrations below this reference value, a situation that may reflect various factors, such as low intake (below 2/3 of the RDA in 87.8% of the patients), altered folate metabolism in uraemia, and decreased intestinal absorption in patients with CRF.25 Patients with CRF being treated with EPO may have increased folic acid requirements because of the increase in erythropoiesis.26
Elevated plasma levels of homocysteine are considered an independent risk factor for vascular disease in nonuraemic patients and those with CRF. Folic acid administration can decrease homocysteine levels by 30-50%, but only some patients attain normal levels of homocysteine in plasma, and treatment has been shown to be more effective when combined with vitamin B6 and B12 .1
We found only one patient with vitamin B6 deficiency, four with vitamin B12 deficiency and 39 with folic acid deficiency. These numbers suggest that hyperhomocysteinemia is related to low levels of folate, which would favour an increase in the rate of homocysteine production. However, it has recently been reported that homocysteine levels decrease in malnourished patients with end-stage renal disease, and change according to nutrient intake and various other nutritional parameters, indicating that circulating homocysteine levels can become an expression of nutritional status.27 Lower homocysteine levels in patients with end-stage renal disease have been associated with a worse clinical outcome.28 In the light of these considerations, the absence of changes in plasma homocysteine levels in our patients suggests that they maintained or even improved their nutritional status during the study period.
There is currently a consensus that the benefits arising from healthcare interventions should be assessed taking into account the health-related quality of life during the time of survival. The SF-36 questionnaire13 provides an efficient method for measuring the quality of life from the patient's standpoint, scoring standardized responses to standardized questions. From the application of this questionnaire to our patients, we observed that better results are obtained in the categories of general health and physical state (see Results) among the patients in the experimental group. These results are considered to be the consequence of the fact that the use of these dietetic foods makes it possible to prepare more varied and better balanced meals, and thus fulfil the goal of achieving a low-protein diet.
The results of this study indicate that the consumption of the dietetic products supplied was very well accepted by all patients, who were thus able to better control their protein intake. This improved control was accompanied by an improvement in vitamin B6 levels and a higher quality of life. These findings, together with the absence of changes in plasma homocysteine levels, indicate that the nutritional intervention had beneficial effects. However, the dietetic products will need to be tested for longer follow-up periods to determine the extent of potential improvements to be attained from low-protein dietetic foods.
References
1. Chazot C, Kopple JD. Vitamin metabolism and requirements in renal disease and renal failure. In Kopple JD, Massry SG, (eds): Nutritional Management of Renal Disease. Philadelphia, PA: Lippincott Williams. 2004; pp. 315-56. [ Links ]
2. Beto JA, Fada Rd, Bansal VK. Medical nutrition therapy in chronic kidney failure: Integrating clinical practice guidelines. J Am Diet Assoc 2004; 104: 404-9. [ Links ]
3. Johnson DW. Dietary protein restriction as a treatment for slowing chronic kidney disease progression: The case against. Nephrology 2006; 11: 58-62. [ Links ]
4. Bostom AG, Lathrop L. Hyperhomocysteinemia in end--stage renal disease: prevalence etiology, and potential relationship to arteriosclerotic outcomes. Kidney Int 1997; 52: 10-20. [ Links ]
5. Sánchez C, Planells E, Aranda P, Perez de la Cruz A, Asensio C, Mataix J, Llopis J. Vitamin B complex and homocysteine in chronic renal failure. Nutr Hosp 2007; 22: 661-71. [ Links ]
6. Kopple JD. Nutritional management of nondialyzed patients with chronic renal failure. In Kopple JD, Massry SG, (eds): Nutritional Management of Renal Disease. Philadelphia, PA: Lippincott Williams. 2004; pp. 379-414. [ Links ]
7. Levey AS, Adler S, Greene T. Effects of dietary protein restriction on the progression of moderate renal disease in the Modification of Diet in Renal Disease Study. J Am Soc Nephrol 1996;7: 2616-26. [ Links ]
8. Kopple JD. National Kidney Foundation K/DOQI Clinical Guidelines for Nutrition I Chronic Renal Failure. Am J Kidney Dis 2001; 37: S66-S70. [ Links ]
9. K-DOQI. Clinical practice guidelines for nutrition in chronic renal failure. National Kidney Foundation. Am J Kidney Dis 2000; 35: S1-S140. [ Links ]
10. American Dietetic Association. Nutrition management of chronic renal insufficiency. In Chicago Dietetic Association and South Urban Dietetic Association (ed.); Manual of Clinical Dietetics. Chicago: pp. 521-34, 1996. [ Links ]
11. Cameron ME, Van Staveren WA. Manual on Methodology for Food Consumption Studies. Oxford: Oxford University Press, 1988; p. 83. [ Links ]
12. Mataix J, Mañas M, Llopis J, Martínez de Victoria E, Sánchez J, Obregón A. Tablas de composición de alimentos españoles (Spanish Food Consumption Tables). Granada, Spain: Editorial Universidad de Granada, 1998. [ Links ]
13. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992; 30: 473-83. [ Links ]
14. Vuilleuimier JP, Keller HE, Rettenmaier R, Hunzinker F. Clinical chemical methods for the routine assessment of the vitamin status in human populations. Part II: The water-soluble vitamins B1, B2 and B6. Intl J Vit Nutr Res 1983; 53: 359-70. [ Links ]
15. Sauberlich HE. "Laboratory tests for the assessment of nutritional status." Boca Raton, FL: CRC Press, 1999. [ Links ]
16. Ihle BU, Becker GJ, Whitworth JA, Charlwood RA, Kincaid-Smith PS. The effects of dietary protein restriction on the progression of renal insufficiency. N Engl J Med 1989; 321: 1773-7. [ Links ]
17. Fassbinder W. Nutrition in chronic kidney disease. Medizinische Wel 2006; 57: 232-8. [ Links ]
18. Maroni BJ. Requirements for protein, calories and fat in the predialysis patient In Mitch WF, Klahr S. (eds.) Nutrition and the kidney, 2nd ed. Boston: Little, Brown, 1993; pp. 185-212. [ Links ]
19. Kopple JD, Berg R, Houser H, Steinman TI, Teschan P. Nutritional status of patients with different levels of chronic renal insufficiency. Kidney Int 1989; 36 (Suppl. 27): S184-94. [ Links ]
20. Mataix J, López-Frías M, Martínez de Victoria E, López-Jurado M, Aranda P, Llopis J. Factors associated with obesity in an adult Mediterranean population: Influence on plasma lipid profile. J Am Coll Nutr 2005; 24: 456-65. [ Links ]
21. Mydlik M, Derzsiova K. Erythrocyte vitamin B1, B2 and B6 in nephrotic syndrome. Miner Electrolyte Metab 1992; 18: 293-4. [ Links ]
22. Planells E, Sánchez C, Montellano MA, Mataix J, Llopis J. Vitamin B6 and B12 and folate status in an adult Mediterranean population. Eur J Clin Nutr 2003; 57: 777-85. [ Links ]
23. Kopple JD, Mercurio K, Blumenkrantz MJ, Jones MR, Tallos J, Roberts C, Card B, Saltzman R, Casciato DA, Swendseid ME. Daily requirements for pyridoxine supplements in chronic renal failure. Kidney Int 1981; 19: 694-704. [ Links ]
24. Gilmour ER, Hartley GH, Goodship THJ. Trace Elements and Vitamins in Renal Disease. In Mitch WE, Klahr S (eds.): Handbook of Nutrition and the Kidney. Philadelphia: Lippincott-Raven, 1998; pp. 107-22. [ Links ]
25. Said HM, Vaziri ND, Karinger RK, Hollander D. Intestinal absorption of 5-methyltetrahydrofolate in experimental uremia. Acta Vitaminol Enzymol 1984; 6: 339-46. [ Links ]
26. Mydlik M, Derzsiova K. Vitamin levels in the serum and erythrocytes during erythropoietin therapy in hemodialyzed patients. Bratisl Lek Listy1999; 100: 426-31. [ Links ]
27. Garibotto G, Sofia A, Valli A, Tarroni A, Di Martino M, Cappelli V, Aloisi F, Procopio V. Causes of hyperhomocysteinemia in patients with chronic kidney diseases. Semin Nephrol 2006; 26: 3-8. [ Links ]
28. Suliman ME, Stenvinkel P, Bárány P, Rashid Qureshi A, Lindholm B. Hyperhomocysteinemia, malnutrition, and inflammation in ESRD patients. Semin Nephrol 2006; 26: 14-9. [ Links ]
![]() Correspondence:
Correspondence:
Cristina Sánchez.
Departamento de Fisiología.
Facultad de Farmacia. Campus Cartuja.
Universidad de Granada
18071 Granada. Spain.
E-mail: crissg@ugr.es
Recibido: 25-III-2009.
Aceptado: 15-VI-2009.