My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.25 n.3 Madrid May./Jun. 2010
Influence of flaxseed during lactation on the reproductive system of female Wistar rats
Influencia de la linaza durante la lactancia sobre el sistema reproductivo de ratas Wistar
L. Leal Soares1, L. Ferreira Medeiros de França Cardozo1, A. Andrade Troina2, C. De Fonte Ramos3, M.a A. Guzmán-Silva4 and G. Teles Boaventura1
1Laboratory of Experimental Nutrition. Department of Nutrition and Dietetic. Nutrition College. Fluminense Federal University (UFF). Niterói. Brazil.
2Laboratory of Endocrine Physiology. Rio de Janeiro State University (UERJ). Rio de Janeiro. Brazil.
3Urogenital Research Unit UERJ. Rio de Janeiro. Brazil.
4Department of Pathology UFF. Niterói. Brazil.
ABSTRACT
Objetives: The goal of this article was to evaluate the association between flaxseed intake during lactation and its effects on the reproductive indexes in female offspring at infancy, puberty and adult age.
Material and methods: Two groups were evaluated, an experimental group (FG, n = 24) which consumed a flaxseed based diet and a control group (CG, n = 26) which had access to a casein based diet. Both of them were fed exclusively with the mentioned diets during all lactation and after weaning the pups received a standard laboratory diet until sacrifice (at weaning, in the moment of vaginal opening or at 90 days old). It was analyzed the puberty onset, estrous cycle, serum estradiol and albumin concentrations, body weight, uterine and ovarian weights.
Results: Estradiol and albumin serum concentrations, body weight, uterine and ovarian relative weights were similar in FG and CG at weaning, at vaginal opening and at 90 days old. There was not significant difference in puberty onset between FG and CG, both had similar body weight at vaginal opening. The length of estrous cycle was similar for both groups. There was no significant difference concerning number of females with irregular estrous cycle, only 2 females had irregular cycle in FG and 3 in the CG. None of the females was acyclic.
Conclusion: Flaxseed intake during lactation did not interfere with sexual maturation and reproductive organs development, suggesting that its consumption during this period is safe for sexual development of female offspring.
Key words: Flaxseed. Uterus. Ovary. Puberty. Estrous cycke.
RESUMEN
Objetivos: el propósito de este artículo era evaluar la asociación entre la ingestión de hierba del chancho durante la lactancia y sus efectos sobre los índices de reproducción en las hijas en la lactancia, pubertad y edad adulta.
Material y métodos: se evaluaron dos grupos, uno experimental (FG, n = 24) que consumía una dieta basada en la hierba del chancho y un grupo control (CG, n = 26) que consumía una dieta basada en caseína. Ambos grupos fueron alimentados exclusivamente con las dietas mencionadas durante toda la lactancia y tras el destete los cachorros recibieron una dieta estándar de laboratorio hasta su sacrificio (en el destete, en el momento de la apertura vaginal o hasta la edad de 90 días.) Se analizó el inicio de la pubertad, el ciclo estrogénico, el estradiol sérico y las concentraciones de albúmina, el peso corporal, y los pesos del útero y los ovarios.
Resultados: las concentraciones séricas de estradiol y albúmina, el peso corporal y los pesos relativos del útero y los ovarios fueron similares entre FG y CG en el destete, en el momento de la apertura vaginal y a los 90 días de vida. No hubo una diferencias significativas en el inicio de la pubertad entre FG y CG, y ambos grupos mostraron un peso corporal similar en el momento de la apertura vaginal. La duración del ciclo estrogénico fue similar en ambos grupos. No hubo una diferencia significativa con respecto al número de hembras con ciclos estrogénicos irregulares; sólo 2 hembras tuvieron un ciclo irregular en el FG y 3 en el CG. Ninguna hembra se quedó sin ciclo.
Conclusión: el consumo de hierba del chancho durante la lactancia no interfirió con la maduración sexual ni el desarrollo de los órganos reproductores, lo que sugiere que su consumo durante este periodo es seguro para el desarrollo sexual de los descendientes femeninos.
Palabras clave: Hierba del chancho. Útero. Ovario. Pubertad. Ciclo estrogénico.
Introduction
Flaxseed (Linum usitatissimum) is an oilseed widely used as a food complement, source of soluble and insoluble fibers, protein (presenting 20% in its composition), 41% fat, 6% of carbohydrate and 4% of residue.1 This seed is regarded as a functional food; this term is applied to foods or ingredients which, besides having basic nutrition functions produce metabolic and physiologic effects which are beneficial to health, when consumed as part of the usual diet.2 Literature shows beneficial effects of flaxseed intake in many situations such as in the prevention of cardiovascular diseases, in the reduction of cholesterol and triglycerides levels and improvement of lipid profile3; in the control of menopause symptoms4; in the prevention of osteoporosis5 and breast and uterine cancer, which grow under the influence of hormones.6
Flaxseed is known as the biggest source of lignans, compounds with chemical structure similar to estrogen. This resemblance suggests that these phytocompounds may bind to estrogen receptors, acting as agonists or antagonists, depending on the amount present in the organism. Under normal estrogen concentrations conditions, which occurs in pre-menopause women, lignans act as antagonists due to its capacity of promoting a negative feedback, reducing the action of this hormone. 7 Conversely, in post-menopause, when estrogen levels are usually lower, lignans act as agonists, acting as estrogens despite being less potent.8
Among the major estrogens, estradiol is the most important and abundant in circulation. In serum, estrogens, as well as other hormones, circulate either as free molecules or protein-bound. In humans, nearly all of the estradiol present in serum is bound to albumin (61%) and sex hormone binding globulin (SHBG) (37%), whereas only a small amount circulates freely (1.5%). The estradiol that circulates through the body in the free or non-protein bound state is considered to be "biologically" active. The estradiol bound to SHBG is not biologically available to tissues, except for the liver, which has a specific receptor for the SHBG-estradiol complex. The estradiol bound to albumin dissociates rapidly for uptake by all tissues with estradiol receptors.9
Although many benefits have been attributed to flaxseed intake, its consumption during critical periods of development such as gestation and lactation is risky due to its phytoestrogens content. During these stages, minimal nutritional or hormonal alterations can yield permanent modifications in the body, with adverse consequences in early life or later, at adolescence or adult age, being this phenomenon termed metabolic imprinting.10
Previous studies report that female offspring from rats that received diets with 10% of flaxseed during gestation and lactation presented alterations in the puberty onset, in uterine and ovarian weight, and longer estrous cycle with lengthened estrus phase.11 On the other hand, the supplementation with 5 or 10% of flaxseed during gestation and lactation resulted in prolongation of estrous cycle with lengthened diestrus and higher percentage of irregular cycle or acyclic rats.12 Rats whose dams received a diet supplemented with a high concentration of flaxseed (40%) during gestation and lactation did not present difference in puberty onset and body weight when compared to control group, whose mothers received casein based diet. However, it was observed significant decrease in the number of females with regular cycles. It was expected that cases of interruption of estrous cycle should occur due to the high concentration of flaxseed used in thisstudy. However, no acyclic females were found.13
Little is known about the use of flaxseed exclusively during lactation. The majority of studies available in the literature used this seed in the two periods, gestation and lactation, and are rather inconclusive. These exhibit contradictory results, varying according to the concentration used.11,12,13
The present study aims to evaluate, for the first time, the effects of 25% of flaxseed during lactation upon puberty onset of the female offspring, estrous cycle, serum estradiol and albumin concentrations, body weight, uterine and ovarian weight at infancy, adolescence and adult age.
Material and methods
Animals
Female Wistar rats, aged 90 days, from the Experimental Nutrition Laboratory (LABNE) of the Department of Nutrition and Dietetic, Nutrition College, Fluminense Federal University (Niterói, RJ, Brazil) were caged with one male at a proportion of 2:1, receiving a standard laboratory diet (Nuvilab, Nuvital Nutrientes Ltda., Colombo, PR, Brazil) during the mating. Animals were kept in polypropylene cages, in a room with controlled temperature (25 ± 1oC) and artificial dark/light cycle (lights on from 7:00 am to 7:00 pm), receiving food and water ad libitum throughout the experiment. The use and handling of experimental animals followed the principles described in the norms from Brazilian College of Animal Experimentation (COBEA). The project was approved by the Ethical Committee for care and use of laboratory animals from Federal Fluminense University (UFF).
Experimental protocol
After delivery, dams were randomly divided in two groups: Flaxseed Group (FG), fed a casein-based diet added to 25% flaxseed and Control Group (CG), fed a casein-based diet. At this moment, excess pups were removed so that only six female pups were kept per dam, as it has been shown that this procedure maximizes lactation performance.14 Dams received their respective isoproteic (17% protein) and isocaloric diets throughout the 21 days of lactation, and food consumption and body weight were monitored daily.
At weaning, one or two pups from each dam were randomly chosen to form the respective groups: FG (n = 24) and CG (n = 26). They received a standard laboratory diet and this was maintained until they were sacrificed; 8 to 10 animals from each group were sacrificed at weaning (infancy), at vaginal opening (puberty) and at 90 days old (adult age) in metestrus stage of estrous cycle.
From day 30 onwards, the females were inspected daily for vaginal opening (VO). Onset of puberty was defined as the age (in days) in which VO occurred.15 Cyclic stages of the ovaries were evaluated by daily vaginal smears after vaginal opening until 90 days old.
After each evaluated period, animals were anesthetized, had blood collected by cardiac puncture to determine serum estradiol and albumin concentrations, and were sacrificed with a lethal dose (0.15 ml/100g body weight, i.p.) of thiopental (Cristália Pharmaceutical Chemical Products LTDA, Rio de Janeiro, RJ, Brazil). The uterus and ovaries were removed and weighted in analytic scale (Bosch S200, Campinas, SP, Brazil) with 0.0001g accuracy.
Diets
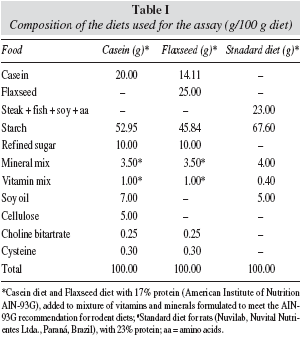
The supplier of components of the diets were: flaxseed (Arma Zen Natural Products Ltda., Rio de Janeiro, RJ, Brazil); starch (Maizena, Unilever Brazil Ltda., Mogi Guaçu, SP, Brazil); refined sugar (União, Rio de Janeiro, RJ, Brazil); soy oil (Liza, Cargill Agricultura Ltda., Mairinque, SP, Brazil); cellulose (Microcel, Blanver Ltda., Cotia, SP, Brazil); cysteine, choline bitartrate and mixtures of vitamins and minerals (Rhoster Commerce and Industry Ltda., Vargem Grande Paulista, SP, Brazil).
The flaxseeds were ground until the flour was obtained, which was used in the preparation of the experimental diet. In the flaxseed diet, it was not necessary to add oil and fiber (cellulose) because this seed is source of these components.1
The ingredients of the diets were weighted in digital scale (Toledo, São Paulo, SP, Brazil) with 0.1g accuracy and were homogenized in a industrial mixer with boiling water. The mass obtained is transformed into tablets, which were dried in a ventiled oven (Fabbe-Prima, São Paulo, SP, Brazil) at 60oC for 24h, properly identified and stored under refrigeration until the time for use. FG and CG diets were prepared in LABNE as demonstrated in table 1 and contained 17% protein (AIN-93G), added to mixtures of vitamins and minerals according to the rules of the Committee on Laboratory Animal Diets, modified according to the recommendations of the American Institute of Nutrition-93.16 In the standard laboratory diet the principle protein sources are steak, fish, soy and amino acids (table I).
Chemical centesimal composition of the diets
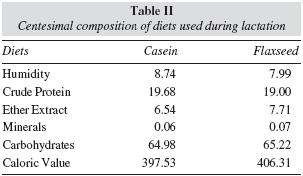
Table II shows the chemical analyses of the diets, made in triplicate, being the average registered. Determination of humidity, total lipids, minerals and protein were performed according to Association Official Analytical Chemists.17 Humidity was obtained through gravimetry on oven at 105oC until constant weight; a Soxhlet extractor and petrol oil as solvent were used to total lipid extraction; minerals were determined by gravimetry using muffle at 550oC; protein was measured by Micro-Kjeldahl method to total nitrogen, using 6.25 as conversion factor from the nitrogen percentage found; Nifext fraction (nitrogen-free extract) was calculated by the difference between 100 and the sum of the other analysed fractions, obtaining the average percentage of carbohydrate.18
Determination of serum albumin (g/dL) e estradiol (pg/mL) concentrations
Blood samples were centrifuged at 3,500 rpm for 15 min, the serum was separated and stored at -20oC until analysis. Albumin was determined by a colorimetric method using commercial kits (Bioclin, Belo Horizonte, MG, Brazil).
Estradiol concentration was obtained using an automated immunochemiluminescence assay (Immulite DPC H2967, Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). This method is a competitive enzymatic quimioluminescent immunoassay in solid phase, which uses a rabbit polyclonal estradiol antibody conjugated with alkaline phosphatase. The analytic sensibility is 15 pg/mL.
Estrous cycling
From VO until 90 days old, vaginal smears were obtained daily through the introduction of a salinedampened cotton swab in vaginal orifice. The smear was placed on a slide, fixated with alcohol 95%, stained through the Papanicolau's method and examined under an optical microscope (20x power) to determine the stage of estrous cycle, described as proestrus, estrus, metestrus and diestrus.19
The number of days required to complete all four stages was counted as one cycle.20 Irregular cycles were defined as those remaining in one stage for 10 days or more. Rats were considered acyclic if they stopped cycling and remained in one stage for the duration of the experiment.12
Statistical Analyses
Data are reported as means ± SD. The normal distribution of values was tested through Kolmogorov-Smirnov test. Student's T test was used (for data that followed a normal distribution) or non-parametric Mann-Whitney test (for data that did not follow a normal distribution). The acceptable level of significance was set at p < 0.05.
Results
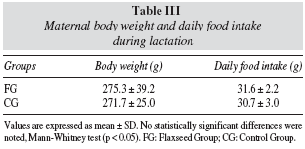
Table III shows that maternal food intake and body weight during lactation were not significantly different among dams which received diet added to flaxseed and control group.
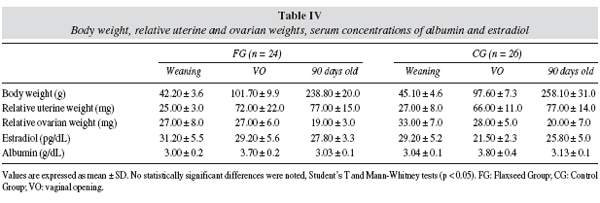
As table IV illustrates, among offspring, estradiol and albumin serum concentrations, body weight, uterine and ovarian relative weights were similar in FG and CG at weaning, at vaginal opening and at 90 days old. There was not significant difference in puberty onset between FG (VO: 34 ± 2 days) and CG (VO: 33 ± 2 days); both groups had similar body weight at vaginal opening.
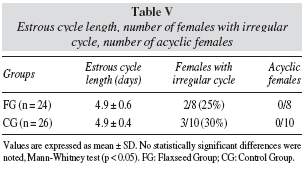
In table V, the length of estrous cycle was similar between FG and CG. There was not significant difference concerning number of females with irregular estrous cycle, only 2 females had irregular cycle in FG and 3 in the CG. None of the females was acyclic.
Discussion
Dietary fibers, among others functions, are capable of accelerating intestinal transit besides promoting satiety sensation, helping to reduce body weight.21,22 In the present study, it was observed that although flaxseed presents high concentration of soluble and insoluble fibers in its composition, it did not interfere with food intake or body weight on the grounds that dams that received flaxseed based diet exhibited similar results when compared to control group.
Modifications of diet during critical periods of development, such as lactation, can yield permanent modifications of the offspring, even if pups have free access to standard laboratory diet after weaning.23 Such effect is known as metabolic imprinting, a definition that was given to the set of biological phenomena that occur due to nutritional experiences in the beginning of life and their relation with disturbances that can occur in the long run, at different ages.24 Countless works report consequences of metabolic imprinting not only in children but also in adults.25,26,27 For that reason, the present study analyzed the effects of flaxseed intake during lactation at different moments: infancy, puberty and adult age.
Serum proteins, especially albumin, have a direct relationship with the type of diet. It was verified that when flaxseed was used during lactation, it had no impact upon albumin concentrations in none of the phases analyzes in this study. It's an utterly important observation given that the majority of circulating estradiol is bound to this protein. Similar results were found when a diet rich in phytoestrogens, such as soy, was given during gestation and lactation. No differences were found concerning the concentrations of albumin between rats fed with soy protein when compared to the ones that received casein.28
Several authors reported that foods rich in phytoestrogen, such as flaxseed or soy, can promote antiestrogenic effects as aromatase inhibition, causing decrease in endogenous estradiol and therefore alterations in reproductive index.6,29,30,31 However, maternal intake of flaxseed during lactation did not alter serum levels of estradiol at the ages analyzed. This fact can justify the results found, as the absence of effects upon body weight and relative weight of uterus and ovaries at the infancy, puberty and adult age. In addition to this, unaltered estradiol levels agree with the absence of interferences in estrous cycle, puberty onset, and body weight at vaginal opening. There is a close relationship between body weight and sexual maturation, suggesting that menarche is triggered by the attainment of a particular body weight, being this factor more important than the age to puberty onset.32,33 Similar results were found when a high concentration of flaxseed (40%) was used in rats during gestation and lactation. As the present study, no differences in relation to the time of vaginal opening, body weight or ovarian weight were found.13
Conversely, opposite results were obtained when the intake of 5% and 10% flaxseed during gestation and lactation were tested. Rats whose dams received 10% flaxseed had earlier age and lighter body weight at puberty, greater uterine and ovarian relative weights and shortened anogenital distance. In contrast, rats whose dams received 5% flaxseed showed delayed puberty by approximately 5 d, reduced immature ovarian relative weight by 29% and tended to lengthen diestrus, indicating an antiestrogenic effect.13
According to the literature, flaxseed can yield different results depending on the concentration used and the period of consumption. Until the present moment, only one study has used flaxseed during lactation exclusively, showing that 10% of this seed did not interfere with puberty onset, estrous cycle length, uterine and ovarian weights and histological analyses of these organs.20
The present study was first one to analyze the effects of 25% flaxseed during lactation and our results corroborated with previous one with different concentration, 20 showing the absence of effects upon reproductive indexes. This observation is of great importance since due these potential health benefits there is increased consumption of foods rich in phytoestrogens by general population, including pregnant and lactating woman and that these compound are transferred through maternal milk and could impair children development.
Flaxseed would be a good option when compared to other phytoestrogens due to the fact that its intake during lactation did not cause harm to offspring's reproductive organs. Conversely, it has been demonstrated that continuously soy intake promoted adverse effects upon reproductive system as a decrease in ovarian follicles and endometrial glands.29
Conclusion
Ours results conclude that the intake of 25% flaxseed during lactation did not interfere with sexual maturation or reproductive organs development, suggesting that its consumption during this period would be safe to the sexual development of rat's female offspring.
References
1. Canadian Grain Commission. Canada Western flaxseed an of yellow flaxseed samples, Canadian Grain Commission, 10, Winnipeg, 2001. [ Links ]
2. Stringheta PC, Oliveira TT, Gomes RC, Amaral MPH, Carvalho AF, Vilela MAP. Políticas de saúde e alegações de propriedades funcionais e de saúde para alimentos no Brasil. Rev Bras Cienc Farm 2007; 43 (2): 181-94. [ Links ]
3. Riediger ND, Othman R, Fitz E, Pierce GN, Suh M, Moghadasian MH. Low n-6:n-3 fatty acid ratio, with fish- or flaxseed oil, in a high fat diet improves plasma lipids and beneficially alters tissue fatty acid composition in mice. Eur J Nutr 2008; 47 (3): 153-60. [ Links ]
4. Patade A, Devareddy L, Lucas EA, Korlagunta K, Daggy BP, Arjmandi BH. Flaxseed reduces total and LDL cholesterol concentrations in Native American postmenopausal women. J Womens Health (Larchmt) 2008; 17 (3): 355-66. [ Links ]
5. Boulbaroud S, Mesfioui A, Arfaoui A, Ouichou A, el-Hessni A. Preventive effects of flaxseed and sesame oil on bone loss in ovariectozed rats. Pak J Biol Sci 2008;11(13):1696-701. [ Links ]
6. Wang L, Chen J, Thompson LU. The inhibitory effect of flaxseed on the growth and metastasis of estrogen receptor negative human breast cancer xenograftsis attributed to both its lignan and oil component. Int J Cancer 2005; 116 (5): 793-8. [ Links ]
7. Chilibeck PD, Cornish SM. Effect of estrogenic compounds (estrogen or phytoestrogens) combined with exercise on bone and muscle massin older individuals. Appl Physiol Nutr Metab 2008, 33 (1): 200-12. [ Links ]
8. Serock MR, Wells AK, Khalil RA. Modulators of vascular sex hormone receptors and their effects I estrogen-deficiency states associated with menopause. Recent Pat Cardiovasc Drug Discov 2008; 3 (3): 165-86. [ Links ]
9. Hayashi T, Yamada T. Association of bioavailable estradiol levels and testosterone levels with serum albumin levels in elderly men. Aging Male 2008; 11 (22): 63-70. [ Links ]
10. Moura EG, Lisboa PC, Passos MC. Neonatal programming of neuroimmunomodulation-role of adipocytokines and neuropeptides. Neuroimmunomod 2008; 115 (3):176-88. [ Links ]
11. Tou JCL, Chen J, Thompson LU. Flaxseed and its lignan precursor, secoisolariciresinol diglycoside, affect pregnancy outcome and reproductive development in rats. J Nutr 1998; 128 (11): 1861-8. [ Links ]
12. Orcheson LJ, Rickard SE, Seidl MM, Thompson LU. Flaxseed and its mammalian lignan precursor cause a lengthening or cessation of estrous cycling in rats. Cancer Lett 1998; 125: 69-76. [ Links ]
13. Collins TFX, Sprando RL, Black TN et al. Effects of flaxseed and defatted flaxseed meal on reproduction and development in rats. Food Chem Toxicol 2003; 41: 819-34. [ Links ]
14. Fisbeck KL, Rasmussen KM. Effect of repeated cycles on maternal nutrition status, lactation performance and litter growth in ad bibtum-fed and chronically food-restricted rats. J Nutr 1987; 117: 1967-75. [ Links ]
15. Faria TS, Ramos CF, Sampaio FJB. Puberty onset in the female offspring of rats submitted to protein or energy restricted diet during lactation. J Nut Biochem 2004; 15: 123-7. [ Links ]
16. Reeves PG, Nielsen FH, Fahey Jr GCF. AIN-93 purified diet of laboratory rodents: final report of the American Institute of Nutrition ad hoc Writing Committee on the Reformulation of the AIN-76A rodent diet. J Nutr 1993; 123: 1939-51. [ Links ]
17. Association of Official Analytical Chemists (AOAC). Official methods of analysis, AOAC, 16, Washington, 1995. [ Links ]
18. Oliveira ECM. Composição centesimal do cogumelo do sol (Agaricus blazet). Rev Univ Alfenas 1999; 5: 169-72. [ Links ]
19. Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estros cycle phases of rats: some helpful considerations Braz J Biol 2002; 62 (4): 609-14. [ Links ]
20. Ward WE, Chen J, Thompson LU. Exposure to flaxssed or its lignan during suckling only or continuously does not alter reproductive indices in male and female offspring. J Toxicol Environ Health A 2001; 64 (7): 567-77. [ Links ]
21. Antal M, Regöly-Mérei A, Biró L et al. Effects of oligofructose containing diet in obese persons. Orv Hetil 2008; 149 (42): 1989-95. [ Links ]
22. Soares LL, Lucas AMM, Boaventura GT. Can organic and transgenic soy be used as a substitute for animal protein by rats? Braz J Med Biol Res 2005, 38 (4): 583-6. [ Links ]
23. Vickers MH. Developmental programming and adult obesity: the role of leptin. Cur. Opin Endocrinol Diabetes Obes 2007; 14 (1): 17-22. [ Links ]
24. Simerly RB. Hypotalamic substrates of metabolic imprinting. Physiol Behav 2008; 94 (1): 79-89. [ Links ]
25. Charalambous M, da Rocha ST, Ferguson-Smith AC. Genomic imprinting, growth control and the allocation of nutrition resources: consequences for postnatal life. Curr Opin Endocrinol Diabetes Obes 2007; 14 (1): 3-12. [ Links ]
26. Djiane J, Attig L. Role of leptin during perinatal metabolic programming and obesity. J Physiol Pharmacol 2008; 59 (1): 55-63. [ Links ]
27. García-Peláez B, Vilà R, Remesar X. Treatment of pregnant rats with oleoyl-estrone slows down pup fat deposition after weaning. Reprod Biol Endocrinol 2008; 20: 6-23. [ Links ]
28. Wilson TA, Garner SC, Anderson JJB. Dietary protein source and serum estradiol concentrations of rats during pregnancy, lactation and post-lactation. Nutr Res 2000; 20 (12): 1735-47. [ Links ]
29. Brasil FB, Soares LL, Faria TS et al. The impact of dietary organic and transgenic soy on the reproductive system of female adult rat. Anat Rec 2009; 292 (4): 587-94. [ Links ]
30. Ju YH, Doerge DR, Woodling KA, Hartman JA, Kwak J, Helferich WG. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent breast cancer cells (MCF-7Ca) in vivo. Carcinogene sis 2008; 29 (11): 2161-8. [ Links ]
31. Faria TS, Soares LL, Medeiros JL Jr, Boaventura GT, Sampaio FJ, da Fonte RC. Morphological modifications of female bladder after prolonged use of soy-based diets. Maturitas 2008; 62 (1): 42-6. [ Links ]
32. Frisch ER. Body fat, puberty and fertility. Biol Rev Camb Philos Soc 1984; 59: 161-8. [ Links ]
33. Heger S, korner A, Meigen C et al. Impact of weight status on the onset and parameters of puberty: analysis of three representative cohorts from center Europe. J Pediatr Endocrinol Metabol 2008; 21 (9): 865-77. [ Links ]
![]() Correspondence:
Correspondence:
Lavinia Leal Soares.
Fluminense Federal University.
24020-150 Rio de Janeiro. Brazil.
E-mail: lavinialeal@yahoo.com.br
Recibido: 24-VI-2009.
Aceptado: 9-VIII-2009.