My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.25 n.6 Madrid Nov./Dec. 2010
Taurine and glucose metabolism: a review
Taurina y metabolismo de la glucosa: una revisión
C. De la Puerta1, F. J. Arrieta1, J. A. Balsa1, J. I. Botella-Carretero1, I. Zamarrón1 and C. Vázquez1,2
1Unidad de Nutrición Clínica y Dietética. Servicio de Endocrinología y Nutrición. Hospital Universitario Ramón y Cajal. Madrid. 2Jefe de Servicio. España.
ABSTRACT
Introduction: Taurine has probed to be involved in a wide range of biological processes and to provide several different important health benefits. Its effects have been revealed to be exerted mainly through its antioxidant and anti-inflammatory effects, among other mechanisms.
Objectives and methods: The present review is aimed to provide a solid body of evidence regarding the beneficial effects of taurine in the context of diabetes and its complications, with an special focus on the cardiovascular health impairments so frequently associated to this disease, so that data from this updated systematic review of the literature, may constitute a base to back up future clinical and epidemiological studies, on the possibilities of taurine supplementation as a useful tool for both prevention and treatment of diabetes complications.
Conclusions: We consider results from the different experimental, in vitro studies as well as some clinical ones reviewed, to provide sufficient evidence as to constitute a solid base to back up future clinical and epidemiological studies on the usefulness of taurine supplementation both in the prevention and treatment of diabetes and its complications.
Key words: Taurine. Diabetes. Diabetes complications. Cardiovascular health. Oxidative damage. Anti-inflammation.
RESUMEN
Introducción: El aminoácido taurina ha demostrado estar involucrado en una amplia gama de procesos biológicos, así como proporcionar distintos e importantes beneficios para la salud. Los principales efectos de la taurina que han sido puestos de manifiesto a través de distinto estudios, residen principalmente en su acción antioxidante y en su capacidad antiinflamatoria, entre otros que también señalaremos.
Objetivos y métodos: La presente revisión tiene como objetivo proporcionar una sólida evidencia en relación a estos y otros efectos de la taurina en el contexto de la diabetes y sus complicaciones, con especial énfasis en las alteraciones de la salud cardiovascular, tan frecuentemente asociadas a esta enfermedad, de manera que los datos de esta revisión sistemática de la literatura que hemos realizado, puedan constituir un base con la que respaldar futuros estudios clínicos y epidemiológicos, sobre las posibilidades que tiene la suplementación con taurina de convertirse en herramienta útil, tanto en la prevención como en el tratamiento de la diabetes y sus complicaciones.
Conclusiones: Consideramos que los resultados de los distintos estudios experimentales, in vitro y algunos clínicos, proporcionan evidencia suficiente como para servir de base científica a futuros estudios clínicos y epidemiológicos sobre la utilidad de la suplementación con taurina en la enfermedad de diabetes y en sus complicaciones.
Palabras clave: Taurina. Diabetes. Complicaciones diabéticas. Salud cardiovascular. Daño oxidativo. Antiinflamación.
Introduction
Taurine (2-aminoethylsulphonic acid) is a non-protein aminoacid present in almost all animal tissues and the most abundant free intracellular aminoacid in human cells.1
In humans, it is considered to be a "semi-essential aminoacid" since it can be synthesized from other sulfonic aminoacids such as methionine and cysteine, in the presence of vitamin B6,2,3 but endogenous production is insufficient, so that it needs to be provided through diet. Animal food products constitute its unique dietetic supply.4
Due to its unique chemichal structure, Taurine is involved in numerous biological and physiological functions, what gives place to important health benefits. Thus, taurine participates in bile acid formation, it exerts antioxidant and anti-inflammatory actions as well as antiarrhythmic/ionotropic/chronotropic ones,2 besides constituting a central nervous system neuromodulator and being also involved in retinal development and function.
On the other hand, both in children and adults, longterm Parenteral Nutrition (PN) has been associated with hepatobiliary dysfunctions, and since taurine is involved in the formation of bile acid conjugates, it is a likely fact that its deficiency might give place to states of cholestasis that are frequently found associated to prolonged (PN).1,3
In the last years, the beneficial roles of taurine on diabetes have been studied. One of the main vias through which taurine plays a beneficial role in diabetes is through its ability to block toxicity caused by oxidative stress.5 Diabetes has been associated with a decline in the levels of this important endogenous antioxidant in several tissues, what raises the possibility that this decline might negatively contribute to the severity of the oxidant-mediated damage present in the diabetic context. In this paper, we will review experimental, in vitro and clinical studies on the role of taurine on both type 1 and type 2 diabetes as well as its metabolic effects on glucose metabolism and diabetes complications, focusing on the effects of this aminoacid upon the different cardiovascular alterations associated to this disease.2,3
In this paper we aimed to reveal relevant results from experimental, in vitro and clinical studies on the health beneficial effects of taurine, in the context of both type 1 and type 2 diabetes, in order to provide a solid body of evidence upon which future clinical studies could be based, what might end up supporting the hypothesis that taurine supplementation may constitute a useful tool, both in the prevention as well as in the treatment of diabetes and its frequently associated health complications.
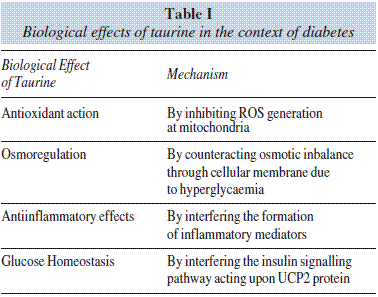
Effects of Taurine on Glucose Homeostasis and metabolism
In the context of diabetes, taurine provides different beneficial effects which are exerted mainly through four different mechanisms of action:
1. Antioxidant activities, specially relevant when exerted at cellular mitochondria.
2. Anti-inflammatory effects.
3. Osmoregulatory actions.
4. Effects on glucose homeostasis.
Antioxidant role of taurine
Although when considering the pathologies derived from diabetes, most studies have focused on the adverse effects of hyperglycemia, it is recently arousing an important number of other studies assessing the role of oxidative stress and damage as the possible link between diabetes and diabetic complications.5 Taurine has demonstrated to play a relevant preventive and therapeutic role through its already proven antioxidant effects which are mainly exerted at mitochondrial level among other cellular and tissular locations, and which constitute its most relevant beneficial action in the context of diabetes.4,5,6 It was Brownlee in 2001 who initially explained that the production of reactive oxygen species (ROS) may be the fact that triggers most of the pathological complications frequently associated to diabetes.5 He thus elaborated the "unifying hypothesis of diabetes", which states that "the generation of superoxide anions in the mithocondria of glucose treated cells, alters key reactions involved in the development of diabetic complications".6,7 (Figure 1)
On the other hand, it has been proven that diabetes is also associated with a decrease in the levels of endogenous antioxidants, particularly of taurine, so that oxidative damage may be enhanced by the deficiency of taurine, since it frequently becomes depleted in diabetic states.5 Although taurine by itself cannot directly scavenge classical ROS (Reactive Oxygen Species) such as superoxide anion, hydroxyl radical, and hydrogen peroxide, this aminoacid has shown to be capable of inhibiting ROS generation.5 Since mithochondrial DNA encodes for the proteins that constitute the electron transport chain, depleted levels of taurine give place to defective tRNas and the subsequent alteration of the translation and expression of these proteins, what at the same time provokes a defective assembly of the respiraroty chain complexes, what leads to a reduction in the transport of electrons through the chain and a diversion of these electrons from the respiratory chain to oxygen, resulting in the formation of the superoxide anion.7 In addition, in 2001, a study by Suzuki et al. using Mass Spectrometric Analysis revealed the presence of two modified uridines in mitochondrial (mt) tRNAs for leucine and lysine. These uridines possess a sulfonic acid group derived from taurine, so that it was for the first time evidenced that taurine is a constituent of biological macromolecules and not only an abundant free aminoacid in several animal tissues.6 One year later, these same authors found these taurine-containing uridines to be lacking, in mutant mitochondrial tRNAS for Leu and Lys from pathogenic cells from patients with mitochondrial encephalomyopathies MELAS and MERRF.8
On the other hand, another of the well established antioxidant actions of taurine is the detoxification of hypochlorous acid, a compound produced by neutrophils as a bactericidal agent.5 Nevertheless, not all of the antioxidant actions of taurine are linked to this detoxification, since some of them have been found in tissues lacking neutrophils.5 There have been revealed other ways through which taurine can act as a powerful antioxidant. In a study by Gurujeyalakshmi et al, results showed that taurine suppresses nitric oxide formation by reducing bleomycin-mediated upregulation of iNOS (inducible nitric oxide synthase).9
Taurine has also demonstrated to exert antioxidant actions by preventing the generation of oxidants. For instance, by disrupting the sequence of events that follow the signalling cascade initiated by toxins what leads to changes in calcium movement, and the subsequent superoxide generation.10 A study by Wu et al illustrates this: in neurons, prolonged N-methyl Daspartate (NMDA) receptor activation by glutamate, gives place to a calcium overload and oxidative stress. In the activation of this receptor, there is an early step consisting in the stimulation of cellular calcium uptake, what gives place to mitochondrial calcium accumulation. This calcium overload damages the mitochondria which in turn generates excessive levels of ROS. According to Wu 2005,10 taurine disrupts this sequence of events by preventing intracellular calcium overload. According to another study by Merezak et al. (2001), Taurine interferes with the action of oxidants through another mechanism consisting in the alteration of cellular membrane fluidity and with this, the activity of key membrane enzymes.11 Taurine exerts this action by altering phospholilpid mehyltranferase activity, an enzyme which determines the phosphatidylethanolamine (PE) and phosphatidylcholine (PC) content of the membrane. As a consequence of this, taurine elevates the PE/PC ratio, what gives place to an alteration of cellular membrane fluidity and the improvement of its ability to resist toxic insults.12
Taurine also protects cells from oxidative stress in an indirect way, by restoring the levels of naturally occuring antioxidants. Several studies have shown that after exposure to different toxics, taurine treatment partially restores the levels of antioxidant enzymes. Among these, a study by Yildirim et al.13 demonstrates this action of taurine upon the thioredoxin reductase activity of aged rats, and another one by Nonaka et al.14 shows the protective action of taurine upon glutathione peroxide activity, in vascular smooth muscle cells from homocystein-treated rats.
Antiinflammatory actions of Taurine
As mentioned above, taurine plays its antioxidant role not by directly scavenging ROS but rather by inhibiting the generation of these and/or by interfering with their oxidant actions. Nevertheless, there is an exception for this, since taurine does have the capability of scavenging a particular compound, hypochlorous acid, an oxidant that activates the tyrosine kinase signalling cascade that leads to the formation of inflammatory mediators.5 Hypochlorous acid is produced in polymorphonuclear leucocytes and eosinophils and works as a bactericidal agent. But when it is produced in excess, it can cause oxidative stress. Both neutrophils and monocytes contain high levels of taurine that can react with hypochlorous acid to form taurinechloramine, which results to be less toxic than hypochlorous acid by itself, so the formation of this taurine conjugate diminishes damage caused by oxidative stress.15 The detoxification of hypochlorous acid used to be considered the only anti-inflammatory action of taurine, but in 2003, subsequent studies by Park et al revealed that taurine-chloramine exerts important anti-inflammatory activities by itself, inhibiting the production of nitric oxide and tumour necrosis factor (TNF-α). This same group had already demonstrated in 1993 that taurine chloramine suppresses the production of IL-6B and IL-8 by polymorphonuclear cells.15 Later on, Kontny et al showed that taurine interfered with an inflammatory cascade by diminishing NF-kB production (Nuclear factor kappabetta), a factor involved in the signalling pathway of pro-inflammatory mediators. In this context it is important to remark that type 1 diabetes is an inflammatory disease, triggered by a neutrophil-mediated destruction of pancreatic -cells, so that it would be of great importance to further investigate the possibility that taurine might lessen the destruction of these cells.16,17
Taurine role as an osmoregulator
Taurine is an important osmoregulator, participating in cell volume regulation together with other low molecular-weighted compounds.1,18 In diabetes, the raised levels of extracellular glucose give place to osmotic stress for cells. In order to counteract the osmotic inbalance across the cellular membrane that occurs in diabetes, either the intracellular production of osmolytes or the transport of external ones is needed. taurine, betaine, myoinositol, sorbitol and glycerophosphorylcholine (GPC) are the most relevant intracellular osmolytes.19 But among them, taurine and betaine have to be transported into the cell since they are not synthesized intracellulary, as it occurs with the others. Taurine plays a key role in the so called "polyol-pathway" (formation of intracellular sorbitol),5,10 due to the fact that this aminoacid has to be transported into the cell by an active specific transporter (TauT), a protein whose expression is osmotically induced, and which is coupled to sodium and choride ions. Its activity is also downregulated in retinal epithelial cells by glucoseinduced PKC activation.20
A study by El-Sherbeny et al provided evidence that hyperosmolarity regulates TauT activity in retinal pigment epithelial cells (RPE) and that TauT is also present in ganglion and Müller cells and it is regulated by hypertonicity. These results are relevant for further studies on the benefits of taurine supplementation in the therapy of retinopathies associated to diabetes, such as macular degeneration, in which retinal cell volume may fluctuate drastically.21 These facts raise the question of whether the depletion of taurine that occurs in diabetes could constitute an explanatory factor of the development of several cellular and vascular dysfunctions associated to this pathology. Nevertheless, this constitutes only a hypothesis, since other low molecular mass compounds found intracellularly might also be involved.18
Glucose homeostasis: effects of taurine on insulin secretion and action
Several studies have revealed that taurine is involved in glucose homeostasis, but the specific molecular mechanisms are unknown.7,22
Taurine exerts effects in glucose homeostais through two known mechanisms:
a) By its effects upon β-cell insulin secretion.
b) By interfering with the insulin signalling pathway and post receptor events.
a) In an experimental study with mice fed with a diet supplemented with and without taurine for 30 days, Carneiro et al obtained results which indicate that taurine controls glucose homeostasis by two mechanisms: by regulating the expression of genes required for the glucose-stimulated insulin secretion and by enhancing peripheral insulin sensitivity. In this study, islets were isolated from taurine supplemented mice and from control ones, and islet cell gene expression and traslocation were examined. Islets from taurine supplemented mice presented: a)-higher insulin content; b)-increased insulin secretion at stimulatory glucose concentrations; c)-slowed cytosolic Ca+2 oscillations in response to stimulatory glucose concentration; d)-increased expression of genes of insulin, sulfonylurea receptor-1, glucokinase, Glut-2, proconverstase and pancreas duodenum homeobox (PDX-1). Besides, mice supplemented with taurine had a significant increased tyrosine phosphorylation of the insulin receptor in skeletal muscle, both at basal and insulinstimulated states.23
Other studies also indicate that taurine exerts hypoglycemic effects by enhancing insulin action,24 as well as by facilitating the interaction of insulin with its receptor.25 On the other hand, taurine increases glycogen synthesis, glycolisis and glucose uptake in the liver and heart of adult rats. These effects were shown to be dependent of insulin concentration.26 In addition, taurine has shown to better ameliorate insulin sensitivity in type 2 diabetes when compared to N-acetylcystein in a study with humans.27
Taurine has demonstrated to exert "mutual stimulating actions with insulin".6 Several reports have shown that plasma taurine levels seem to be important for beta-cell function and insulin action. In a study by Riveiro at al, low concentrations of this aminoacid were found in the plasma of pre-diabetic and diabetic mice.28 In this study, animals were supplemented with 2% (w/v) of taurine added to their drinking water for a period of 30 days. After that time, glucose tolerance and insulin sensitivity were assessed, as well as the insulin secretion from isolated islets stimulated by glucose or L-leucine in both groups: control mice and those receiving taurine. Results of this study show an improved glucose tolerance and a higher insulin sensitivity in mice drinking taurine supplemented water than in the control ones. Moreover, their islets secreted more insulin in response to high concentrations of glucose and L-leucine, and leucine oxidation was higher in the islets from mice having taruine than in those from the control ones. Besides, the L-type beta-2 subunit voltage-sensitive Ca2+ channel protein, as well as the Ca2+uptake, were significantly higher in the taurinesupplemented mice islets than in the ones from control mice. In addition, islets from mice having taurine, secreted more glucagon than those from control ones at low glucose concentrations. This study thus conclude, that supplementation with taurine improves glucose tolerance and insulin sensitivity in mice, as well as insulin secretion from isolated islets. The latter effect seems to be, at least in part, dependent on a better performance by the islet cells.
On the other hand, a study by Nandhini et al.29 showed that the beneficial actions of Taurine on insulin resistance might work by modifying the post-receptor events of insulin action. In this study, high fructose fed rats were used to elucidate whether taurine could improve insulin action by modulating its signal transduction pathway. Rats were divided into 4 groups: one group of control ones received a designed diet and water ad libitum. A second group received high fructose diet (> 60% of total calories) and water ad libitum. A third group of rats was fed on a fructose diet and 2% taurine solution also ad libitum. And finally the fourth group received control diet and 2% taurine solution ad libitum. After a period of 30 days, the effects of taurine on the activities of the hepatic enzymes protein tyrosine kinase (PTK) and protein tyrosine phosphatase (PTP) were assayed. Taurine administration improved insulin sensitivity and controlled hyperglycemia and hyperinsulinemia in fructose-fed rats as well as it restored the glucose metabolizing enzyme. Besides, Taurine has also demonstrated to have hypoglycemic effects during experimental insulin-dependent diabetes mellitus, producing a decrease in the concentrations of glucose and fructosamine as well as an increase in the contents of insulin, C-peptide, and glycogen in the liver. In this experiment, studies on the dynamics of structural changes in the pancreatic tissue confirmed a positive effect of taurine on beta-cell function, showing the protective effect of taurine on insulin-dependent diabetic rats.30
b) Taurine interference with insulin signalling pathway. In addition to all these studies, results from other ones show that taurine acts on several stages of the so called stimulus-secretion coupling process. Going back to the potential role of taurine at mithocondria of cells overexposed to glucose: oxidative mitochondrial metabolism plays a key role in the generation of the signalling cascade which couples glucose recognition to insulin secretion, in pancreatic -cells. Prolonged exposure of these cells to high concentrations of glucose generates oxidative stress, which ends up in -cell dysfunction and in some cases even in cell death.31 Within the mitochondrial inner membrane carrier family, there is a key protein called "Uncoupling protein 2" (UCP2) which catalyzes a proton leak and subsequently hypopolarizes the mitochondrial membrane potential and reduces the cellular content. UCP2 is upregulated in pancreatic beta cells when exposed to prolonged high glucose or free fatty acids, what results in impaired glucose-induced insulin secretion (GIIS). On the other hand, GIIS, which is critical for maintaining normal blood glucose, becomes suppressed when UCP2 is overexpressed.31 In this study by Han et al, the effects of taurine on impaired glucose responses of diabetic rat -cell adenovirally overexpressing UCP2 were studied, which on the other hand is also upregulated in obesity-related type 2 diabetes. Authors found that restoration of plasma levels of taurine could be critical in preventing and/or improving obesity-related -cell dysfunction as well as many other diabetic complications.
Nevertheless, there is a study by Brons et al with opposite results. Authors assessed the effect of taurine treatment on both insulin secretion and action and on plasma lipid levels, in overweightedmen with a positive historiy of type 2 diabetes. In this study, 20 nondiabetic subjects were included in a double-blinded, randomized, crossover study, receiving a daily supplemmentation of 1.5 g of taurine or placebo, for a period of 8 weeks. Subjects were overweighted ones and first-degree relatives of type 2 diabetes patients. An intravenous glucose tolerance test (IVGTT) was used to measure first-phase insulin secretory response, and euglycemic hyperinsulinemic clamp was used to determine peripheral insulin action. There was no significant difference after taurine intervention compared to placebo in incremental insulin response, neither during IVGTT nor in insulin-stimulated glucose disposal during the clamp. Insulin secretion adjusted for insulin sensitivity was also unchanged, and there was no difference on blood lipid levels between the group receiving placebo and the one which was supplemented with taurine.32
Taurine and diabetes associated pathologies: the cardiovascular complications
As it is well known, a series of pathological complications are associated to diabetes, specially when this disease has been of long-term development. According to de unifying hypothesis of diabetes, the main pathological complications associated to this are due to the oxidative damage that accompanies this pathology, thus, the possible therapeutical and/or preventive effects of taurine are based mainly on its antioxidant properties, although other actions exerted by this aminoacid also contribute to provide health benefits to diabetes patients.5 Although we count with very few clinical trials to back up in vitro and experimental findings, a handful of them have reported that taurine supplementation has beneficial effects on platelet aggregation, nephropathy and retinopathy, as well as on vascular dysfunction and cardiomyopathies, all of them being considered as the main clinical complications of diabetes.18 In addition, recent studies have attributed an important role to taurine in fetal development, particularly in the case of diabetic mothers, since taurine might block the transfer of diabetes from these mothers to their offsprings.33
Taurine has been reported to have nephroprotective properties. These may occur as a result of diminished renal NADPH oxidase activity, what is produced by the increased presence of taurine. Thus, this aminoacid seems to be beneficial for the therapy of both diabetes and diabetic nephropathy: in a study by Winiarska et al,34 the potential benefits of taurine on nephropathy associated to diabetes was investigated in alloxan diabetic rabbits. Animals were fed for three weeks with 1% taurine in its daily drinking water. Histological studies of kidneys were performed in addition to measurement of several blood parameters. Taurine administration to diabetic rabbits resulted in 30% decrease in serum glucose level and the normalisation of diabetes-elevated the rate of renal gluconeogenesis. It also diminished serum urea and creatinine concentrations and abolished hydroxil free radicals accumulation in serum, liver, and kidney cortex. In addition, animals supplemented with taurine exhibited elevated activities of gamma-glutamylcystein syntetase and renal glutathione reductase and catalase. Taurine treatment evoked the normalization of the diabetes-stimulated activity of renal NADPH oxidase. Besides, taurine treatment attenuated both albuminuria and glomerulopathy which are characteristic of diabetes. Authors concluded that taurine seems to be beneficial for the therapy of both diabetes and diabetic nephropathy. These results suggest that taurine has the ability to suppress the progression of diabetic nephropathy through its antioxidant effects. Besides, Higo S. et al have referred that the development of proteinuria and the expansion of mesangial extracellular matrix expansion were both efficiently reduced after taurine supplementation in diabetic rats.26 To the view of these results, taurine administration was potentially expected to be applied in clinical field to retard the development of nephropathy in diagnosed diabetic patients.34
In the context of diabetic retinopathy, Yu et al have reported a decrease in the severity of this diabetic complication due to taurine supplementation, which hypothetically exerts this beneficial effect by the inhibition of glutamate toxicity. The study was performed in rats with induced diabetes, which were fed with and without a supplementation of taurine during 4-12 weeks. The supplementation did not lower plasma glucose concentration, but produced an elevation in taurine content and a decline in the levels of glutamate and gamma-aminobutyric acid (GABA) in diabetic retina (p < 0.05).35
Of all diabetes complications, cardiopathies and vascular dysfunctions are among the most relevant and prevalent ones, if we take into account that 80% of deaths among diabetic patients are due to Cardiovascular Diseases (CVD).36,37,38
Although further clinical trials are needed, we already count with some studies which demonstrate that taurine benefits cardiovascular health in diabetes patients. Some mechanisms have been proposed to explain the means by which taurine exerts protection against CVD, namely, through the formation of bile acid conjugates to produce bile salts, which constitute the main via of excretion of cholesterol and also through its demonstrated ability to reduce oxidative stress and inflammation.39
Taurine benefits cardiovascular health of diabetes patients in different apects:
a) Taurine protects from atherosclerosis in diabetic patients. Some of the experimental and in vitro studies have suggested that taurine, when used as a nutritional supplement, might play a relevant role as a protector against oxidative stress and atherosclerosis development. 37,38 In humans, taurine, as well as glycine, forms conjugates with bile acids (mainly cholic acid) giving place to the bile salts taurocholate and glycholate respectively. The first one is the major bile salt that extracts cholesterol from plasma.38 On the other hand, oral administration of taurine has shown to increase relative amounts of taurocholic acid in the bile, whereas no effect on bile acid composition was observed when the dietary supplemment administered was glycine.39 Between 5% and 10 % of the bile acids are excreted via faeces, thus bile salts are only partially reabsorbed. Due to all these facts, we can consider taurine as a relevant player in cholesterol metabolism, since the excretion of bile salts via faeces, constitutes the only excretion route of cholesterol from the body, with a daily output of bile acids of about 200-1,000 mg.40 This means that low levels of taurine in the diet might give place to lower cholesterol extraction and subsequently to its accumulation in plasma, a fact that is well known to substantially increase the risk of developing atherosclerosis.2,4,22
b) Taurine supplementation gives place to an improved lipidic profile. In a study by M. Zhang et al in which the effects of taurine supplemmentation on the lipidic profile of overweighted subjects was studied. Thirty participants, with a Body Mass Index (BMI) > 25 kg/ m2 were supplemented with 3g/day of taurine for 7 weeks, while the control group received placebo. Measurements of triacylglicerol (TG), total cholesterol (TC) and high-density lipoprotein cholesterol (HDLC) were taken, before and after the intervention period, existing no difference at baseline in these parameter levels between groups. The group that recieved taurine supplementation presented a significant decrease in TG (of 8 mg/dl) while the group that had placebo presented an increase of 3g/dl in this parameter. These changes were statistically significantly different between the two groups (taurine and placebo), being p = 0.04.41
These results suggest that taurine does produce an improvement in lipidic profile after supplementation. At another study with twenty two healthy male japanese, aged 18-25, the effects of 6g/day taurine supplementation during 3 weeks versus placebo were assessed. Participants were placed on a diet specially designed to increase their cholesterol levels. This parameter was significantly increased in the group that received placebo, as well as their LDL-cholesterol, and LDL plasma levels, while the group receiving the taurine supplementation suffered significantly smaller increases in these parameters.42
Nevertheless, we must remark that results from these studies, although participant subjects were obese or overweighted, they were not neither diabetes patients nor having any evidence of diabetes at all. But results show the beneficial effects of taurine upon lipidic profile and subsequently upon cardiovascular health, being both key facts in the development of diabetes complications.4,5,6
c) Taurine exerts a protective effect on endothelial dysfunction. As it is well known, endothelial dysfunction is a precursor of atherosclerosis. On the other hand, negative changes in the vascular endothelium are very frequently associated to diabetes, where both hyperglycemia and dislipemia contribute to alter this tissue.43 More specifically, hyperglycemia is considered to be the major casual factor in the development of endothelial dysfunction in diabetes patients, mainly through the formation of advanced glycation end products (AGEs), a biochemical situation that usually appears accompanying diabetes,44 but also through a series of other mechanisms, such as the impairment of the nitric oxide (NO) production cells: in this case, taurine has demonstrated to exert an anti-infammatory action through the previous formation of the compound taurine-chloramine, which inthibits the production of NO and prostaglandin E2 (PGE2), by suppressing inducible nitric oxide synthase and cyclooxygenase, two enzymes which become activated by hyperglycemia. 45 Other mechanisms through which hyperglycemia leads to endothelial dysfunction are an increase in the production of vasoconstrictor prostaglandins, platelet and vascular growth factors, among other cardiovascular phenomena, all of them leading to the onset and development of subsequent atherosclerosis.38,43 In this context, a study with rats showed that microvascular inflammatory injuries caused by hyperglycemia, became reverted after supplementation with taurine, what suggests that this aminoacid may play a role in reducing these effects, by attenuating excess leucocyte activity through the induction of the the formation of less toxic inflammatory mediators.44
As we have stated above, antioxidation is the most relevant biological action through which taurine exerts beneficial effects on diabetes patient health. In the context of cardiopathies associated to diabetes, since taurine is considered to be an effective endogenous antioxidant, it can improve vascular endothelial dysfunction caused by oxidative stress. In a study by Wang et al. in the context of induced experimental type 1 diabetes in rats, authors showed that this effect might be associated with downregulation of the expression of genes that encode for LOX-1 (a novel endothelial receptor for oxidized low-density lipoprotein which might mediate endothelial dysfunction) as well as those for soluble intracellular adhesion molecule-1 (sICAM-1) on aortic vascular endothelium via taurine antioxidative properties. 45,46 This study was aimed to investigate the protective effect of taurine on early vascular endothelial dysfunction and its possible mechanism, by detecting the changes of oxidized-Low Density lipoprotein (oxLDL)/LOX-1 system, in young STZ-induced diabetic rats. In this, rats were divided into three groups (CN group, n = 8), diabetes mellitus group (DM group, n = 8) and taurine supplemented group (DM+TAU group, n = 8). Diabetes was induced in the rats by intraperitoneal injection of streptozotocin (STZ), (60 mg/kg) and after the onset of diabetes, the rats in DM+TAU group were given free access to drinking water containing 1% taurine. At the end of 4 weeks, blood glucose, serum total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL) and highdensity lipoprotein (HDL), oxidized low density lipoprotein (oxLDL) and sICAM-1 levels were determined, meanwhile LOX-1 and ICAM-1 expression on abdominal aortas were examined by immunostaining, Western blotting and reverse transcription PCR, respectively. Compared to normal control, in STZ-induced diabetic rats, the levels of serum TC, TG, LDL, oxLDL and sICAM-1 were all increased (p < 0.01) meanwhile LOX-1 and ICAM-1 expression (protein and mRNA) in the endothelium layers of abdominal aortas were also markedly enhanced (p < 0.01 for all); while in taurine supplemented rats, were all markedly lower than those of untreated diabetic rats (p < 0.05 for all). Also, the level of LOX-1 protein expression was positively correlated with levels of serum oxLDL (r = 0.922, p = 0.001), sICAM-1 (r = 0.753, p = 0.031) and ICAM-1 expression on abdominal aorta (r = 0.849, p = 0.008). Authors concluded that vascular endothelial dysfunction was present in early stage of young diabetic rats and that taurine supplement could protect against this early endothelial dysfunction by its antioxidant action, consisting of the inhibition of the role of oxLDL/LOX-1 system in young rats with diabetes mellitus.47
Another study using human umbilical cord venous endothelial cells, reports that taurine supplementation reduces the expression of molecules such as vacular cell adhesion molecule-1 (VCAM-1), ICAM-1 and its soluble form sICAM-1 also caused by hyperglycaemia In this study, cells were cultivated and exposed to a high glucose concentration medium (60 µ/ml) alone, and in the presence of taurine (0.5-2.5 mg/ml) for 20 hours. Results were given as a percentage of the low glucose medium used as control. As expected, hyperglycemia increased cell-surface expression of VCAM-1, ICAM-1 and sICAM-1, while endothelial cells cultured with added taurine, presented values restored to normal levels in the expression of these molecules, as well as those of oxidized-LDL.48
d) Taurine has demonstrated to lower homocystein plasma levels:an independent marker of cardiovascular risk. Plasma Homocysteine (Hcy) has lately been used as an independent cardiovascular risk predictor.49 On the other hand, a study by Ahn et al evidenced that taurine supplementation has an effect on Hcy levels. Participants were 22 healthy middle-aged women (33-54 years). After 4 weeks of supplementation with 3 g of taurine per day, plasma taurine concentration was significantly higher (p < 001) while the levels of plasma Hcy significantly decreased after supplementation (p < 0.05).50 These results provide more data to support the idea that taurine might be a beneficial nutrient in preventing cardiovascular diseases which on the other hand, are so frequently associated to diabetes mellitus.
e) Taurine exerts antiaggregant effects in the diabetic patient. It is known that diabetes mellitus patients present an increased platelet activity, what contributes to the development of diabetic complications.18 On the other hand, plasma and platelet taurine concentrations are frequently depleted in these patients.9 Taurine supplementation reduces platelet aggregation in diabetes patients, as demonstrated Franconi et al in a study of 39 patients with insulin-dependent diabetes, and 34 control ones which were all matched for age, sex and by protein-derived daily energy intake. Patients were all supplemented with 1.5 g of Taurine for 90 days. Platelet aggregation induced by arachidonic acid was assayed in vitro at base line, resulting to be lower in diabetic patients than in the control ones (p < 0.01). After the period of taurine supplementation, plasma and platelet taurine concentration resulted significantly increased in the diabetic patients, reaching the normal values of the control group. Besides, the dose of arachidonic acid necessary to provoke platelet aggregation, was significantly lower in the diabetic patients than in the control ones.51 Nevertheless, Spohr et al did not observe any beneficial effect of taurine supplements on platelet aggregation in type 2 diabetic patients.52
f) Taurine has positive effects on Blood Pressure. Taurine is considered to decrease blood pressure (BP) through a mechanism consisting of an interference on the angiotensin II signalling, which is in charge of causing vasoconstriction and the subsequent increase in blood pressure.53 We count with a study by the World Health Organization (WHO), the so called WHOCARDIAC study,54 a multicenter cross-sectional studyin which an inverse correlation between 24 hour-urinary excretion of taurine and BP was found in 755 Han participants and 125 Tibetan ones. In the first population group, the correlation was found between urinary excretion of taurine and diastolic pressure, while in the second, this inverse correlation was present with both the diastolic and the systolic one.
In a double blind placebo controlled trial, 19 borderline hypertensive patients were supplemented with 6 g. of taurine a day, what resulted in a significant decrease of systolic and diastolic BP, while the placebo group suffered no changes in this parameter.55 To all these we can add a study on platelets from both cats and humans, in which it was shown that platelet aggregation was associated to increased platelet levels of taurine and glutathione.56,57 Besides, an in vitro study shows another mechanism which may explain the action through which taurine exerts an hypotensive effect, by inhibiting the production of nitric oxide and prostaglandin E2.45
Conclusions
Taurine health benefits are based mainly on its antioxidant and anti-inflammatory power as well as on its osmoregulator activity in the occurrence of hyperglycemia, on one hand, and on is participation in the formation of bile-acid conjugates (taurocholate) which helps excrete cholesterol, and thus improves the metabolic profile of diabetes patients, both type 1 and type 2. Nevertheless, we must remark that the above mentioned health benefits of taurine have been demonstrated mainly through animal and in vitro studies, so that up to date we count with too scarce clinical studies to evidence that taurine supplementation does provide important health benefits in diabetes patients, not only in preventive perspective, but also as a coadjuvant therapeutic tool. Many more clinical studies are needed.
It is important to remark though, that the use of these supplements constitutes the opening of promising therapeutic and preventive possibilities but that the efficacy and safety of such supplements, the adequate dosis to be utilized, still needs much further investigation in clinical trials. Nevertheless, taurine seems to be specially useful as coadjuvant in the therapy of diabetic complications such as retinopathy, nephropaty and particularly in the field of the cardiovascular health alterations associated to diabetes .
In addition to all this, studies on aminoacid formulas for PN, supplemented with taurine are in course, to assess its potential in preventing and/or ameliorating metabolic and inflammatory alterations in patients requiring PN.
Acknowledgments
To the Instituto Ramón y Cajal de Investigación Sanitaria IRYCIS, and FIS . PI 050681.
C de la Puerta is recipient of a fellowship award from Grifols S.A. (Barcelona, Spain) laboratories for clinical studies about the role of Taurine on parenteral nutrition (2010-2011)
References
1. Kim SJ, Gupta RC, Lee HW. Taurine-diabetes interaction: from involvement to Protection. Curr Diabetes Rev 2007; 3 (3): 165-175. [ Links ]
2. Lourenço R, Camilo ME. Taurine: a conditionally essential amino acid in humans? An overview in health and disease. Nutr Hosp 2002; 17 (6): 262-270. [ Links ]
3. Bouceknooghe T, Remacle C, Reusens B. Is taurine a functional nutrient? Current Opinion in Clinical Nutrition and Metabolic Care 2006; 9: 728-733. [ Links ]
4. Szymanski K, Winiarska K. Taurine and its potential therapeutic application. Postepy Hig Med Dosw (Online) 2008; 62: 75-86. [ Links ]
5. Schaffer SW, Azuma J, Mozaffari M. Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol 2009; 87: 91-99. [ Links ]
6. Suzuki T, Suzuki T, Wada T, Saigo K,Watanabe K. Novel taurine-containing uridine derivatives and mitochondrial human diseases. Nucleic Acids Res Suppl 2001; (1): 257-258. [ Links ]
7. Franconi F, Di Leo MA, Bennardini F, Ghirlanda G. Is Taurine Beneficial in Reducing Risk Factors for Diabetes Mellitus? Neurochemical Research 2004; 29 (1): 143-150. [ Links ]
8. Takeo Suzuki, Tsutomu Suzuki, Takeshi Wada, Kazuhiko Saigo, Kimitsuna Watanabe. Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrialdiseases. EMBO J 2002; 21 (23): 6581-6589. [ Links ]
9. Gurujeyalakshmi G, Wang Y, and Giri S.N. Suppression of bleomycin-induced nitric-oxide production in mice by taurine and niacin. Adv Exp Med Biol 2000; 483: 545-61. [ Links ]
10. Wu H, JIN Y, Wei J, Jin H, Sha D, Wu JY. Mode of action of taurine as a neuroprotector. Brain Res 2005; 1038: 123-131. [ Links ]
11. Merezak S, Hardikar AA, Yajnik CS, Remacle C, Reusens B. Intrauterine low protein diet increases fetal-cell sensitivity to NO and Il-1β. the protective role of taurine. J Endocrinol 2001; 171: 299-308. [ Links ]
12. Hamaguchi T, Azuma J, Schaffer S. Interaction of taurine with methionine: inhibition of myocardial phospholipids mehtyltranferase. J Cardiovasc Pharmacol 2001; 18: 224-230. [ Links ]
13. Yildirim Z, Kilic N, Ozer C, Babul A, Take G. Erdogan D. Aging interventions: effects of taurine in cellular responses to oxidative stress in young and middle-aged rat liver. Ann NY Acad Sci 1995; 1100: 553-561. [ Links ]
14. Nonaka H, Tsujino T, Watari y, Enoto N, Yokoyama M. Taurine prevents the the decrease in expression and secretion of extracellular superoxide dismutase induced by homocystein amelioration of homocystein-induced endoplasmic reticulum stress by taurine. Circulation 2001; 104: 1165-1170. [ Links ]
15. Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils: evidence for hypochlorous acid generation. J Clin Inves 1982; 70 (3): 598-607. [ Links ]
16. Park, E, Alberti J, Quinn M.R., Schuller-Levis G. Taurine chloramines inhibits production of superoxide anion, IL-6 and IL-8 in activated human polymorphonuclear leukocytes. Adv Exp Med Biol 442: 177-182. [ Links ]
17. Kontny AK, Scczepanska K, Kowalczewski J, Kurowska M, Janicha I, Marcinkiewicz J, Maslinski W. The mechanism of taurine chloramine inhibition of cytokine (interleukin-6, interleukin-8) production by rheumatoid arthritis. Arthritis Rheum 2000; 43: 2169-2177. [ Links ]
18. Hansen SH. The role of taurine in diabetes and the development of diabetic Complications. Diabetes Metab Res Rev 2001; 17: 330-346. [ Links ]
19. Pasentes-morales H, Schousboe A. Role of taurine in osmoregulation in brain cells: mechanisms and functional implications. Amino Acids 1997; 12: 281-292. [ Links ]
20. Stevens MJ, Hosaka Y, Masterson JA, Jones SM, Thomas TP, Larkin DD. Downregulation of the human taurine transporter by glucose in cultured retinal pigment epithelial cells. Am J Physiol 1999; 277: E760-E771. [ Links ]
21. Amira El-Sherbeny, Hany Naggar, Seiji Miyauchi, M. Shamsul Ola, Dennis M Maddox, Pamela Moore Martin, Vadiviel Ganapathy, Sylvia B. Smith. Osmoregulation of Taurine transporter Function and Expression in Retinal Pigment Epithelial, Ganglion and Müller Cells. Investigative Ophthalmology & Visual Science. 2004. Vol 45, No 2. [ Links ]
22. Kulakowski EC, Maturo J. Hypoglycemic properties of taurine: not mediated by enhanced insulin release. Biochem Pharmacol 1984; 33: 2835-2838. [ Links ]
23. Carneiro EM, Latorraca MQ, Araujo E, Beltrá M, Oliveras MJ, Navarro M, Berná G, Bedoya FJ, Velloso LA, Soria B, Martin F. Taurine supplementation modulates glucose homeostasis and islet function. Journal of Nutritional Biochemistry 2009; 20: 503-511. [ Links ]
24. Lapson WG, Kramer JH, Schaffer SW. Potentiation of the actions of insulin by taurine. Can J Physiol Pharmacol 1983; 61: 457-463. [ Links ]
25. Maturo J, Kulakowski EC. Taurine binding to the purified insulin receptor. Biochem Pharmacol 1988; 37: 3755-3760. [ Links ]
26. Higo S, Miyata S, Jiang QY, Kitazawa R, Kitazawa S, Kasuga M. Taurine administration after appearance of proteinuria retards progression of diabetic nephropathy in rats. Kobe J Med Sci 2008; 54 (1): E35-45. [ Links ]
27. Xiao C, Giacca A, Lewis GF. Oral taurine but not N-acetylcysteine ameliorates NEFA-induced impairment in insulin sensitivity and beta cell-function in obese and overweight, non-diabetic men. Diabetología 2008; 51: 139-146. [ Links ]
28. Ribeiro RA, Bonfleur ML, Amaral AG, Vanzela EC, Rocco SA, Boschero AC, CarneiroEM.Taurine supplementation enhances nutrient-induced insulin secretion in pancreatic mice islets. Diabetes Metab Res Rev 2009; 25 (4): 370-379. [ Links ]
29. Nandhini AT, Thirunavukkarasu V, Anuradha CV. Taurine modifies insulin signaling enzymes in the fructose-fed insulin resistant rats. Diabetes Metab 2005; 31 (4 Pt 1): 337-344. [ Links ]
30. Gavrovskaya LK, Ryzhova OV, Safonova AF, Matveev AK, Sapronov NS. Protective effect of taurine on rats with experimental insulin-dependent diabetes mellitus. Bull Exp Biol Med 2008; 146 (2): 226-228. [ Links ]
31. Lee SH, Lee HY, Kim SY, Lee IK, Song DK. Enhancing effect of taurine on glucose response in UCP-overexpressing beta cells. Diabetes Res Clin Pract 2004; 66: S69-74. [ Links ]
32. C Brons, C Spohr, H Storgaard, J Dyerberg, A. Vaag. Effect of taurine treatment on insulin secretion and action on serum lipid levels in overweighted men with genetic predisposition for type II diabetes mellitus. Eur J Clin Nutr 2004; 58(9): 1239-1247. [ Links ]
33. Giusseppe Seghieri, Federica Tesi, Loria Bianchi, Alberto Loizzo, Giuseppe Sacommanni, Giovanni Ghirlanda, Roberto Anichini, Flavia Franconi. Taurine in women with a history of gestational diabetes. Diabetes Research and Clinical Practice 2007; 76: 187-192. [ Links ]
34. Winiarska K, Szymanski K, Gorniak P, Dudziak M, Bryla J. Hypoglycaemic, antioxidative and nephroprotective effects of taurine in alloxan diabetic rabbits. Biochimie 2009; 91 (2): 261-270. [ Links ]
35. Yu X, Xu Z, Mi M, Xu H, Zhu J, Wei N, Chen K, Zhang Q, Wang J, Chen F; Tang Y. Dietary taurine supplementation ameliorates diabetic retionpathy via-anti-excitotoxicity of glutamate in streptozotocin-induced Sprague-Dawley rats. Neurochem Res 2008; 33 (3): 500-507. [ Links ]
36. Pop-Busui, R, Sullivan KA, Van Huysen C, Bayer L, Cao X, Towns R, Stevens MJ. Depletion of taurine in experimental diabetic neuropathy: implications for nerve metabolic, vascular and functional deficits. Exp Neurol 2001; 168: 259-272. [ Links ]
37. Xu Xj, Arneja AS, Tappia PS, Dhalla NS. The potential health benefits of taurine in cardiovascular disease. Exp Clin Cardiol 2008; 13 (2): 57-65. [ Links ]
38. Hadi AR Hadi, Jassim Al Suwaidi. Endothelial dysfunction in diabetes mellitus. Vascular Health and Risk Management 2007; 3 (6): 853-876. [ Links ]
39. Murakami S, Kondo Y, Nagate T. Effects of long-term treatment with taurine in mice fed a high-fat diet: improvement of cholesterol metabolism and vascular lipid accumulation by taurine. Adv Exp med Biol 2000; 483: 177-186. [ Links ]
40. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813-820. [ Links ]
41. Zhang M, Bi LF, Su XL, Da GL, Kuwamori T, Kagamori S. Beneficial effects of taurine on serum lipids in overweightedor obese non-diabetic subjects. Amino Acids 2004; 26: 267-271. [ Links ]
42. Mizushima S, Nara Y, Sawamura M, Yamori Y. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv Exp Med Biol 1996; 403: 615-622. [ Links ]
43. Ulrich-Merzenich G, Zeitler H, Vetter H, Bhonde RR. Protective effects of taurine on endothelial cells impaired by high glucose and oxidized low density lipoproteins. Eur J Nutr 2007; 46: 431-438. [ Links ]
44. Casey RG, Gang C, Joyce M, Bouchier-Hayes DJ. Taurine attenuates acute hyperglycaemia-induced endothelial cell apoptosis, leucocyte-endothelial cell interactions and cardiac dysfunction. J Vasc Res 2007; 44 (1): 31-39. [ Links ]
45. Liu Y, Tonna-DeMasi M, Park E, Schuller-Levis, Quinn MR. Taurine chloramine inhibits production of nitric oxide and prostaglandin E2 in activated C6 glioma cells by suppressing inducible nitric oxide synthase and cyclooxygenase- 2 expression. Brain Res Mol Brain Res 1998; 59 (2): 189-195. [ Links ]
46. Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomiopathy: Mechanisms, diagnosis and treatment. Clin Sci (Lond) 2004; 107; 539-557. [ Links ]
47. Wang LJ, Yu YH, Wang Y, Niu N, Li Q, Guo LM. Effects of early intervention with taurine on oxLDL/LOX-1 system and vascular endothelial dysfunction in young streptozotocininduced diabetic rats. Zhonghua Er Ke Za Zhi 2009; 47 (3): 194-199. [ Links ]
48. Ulrich-Merzenich G, Zeitler H,Vetter H, Bhonde RR: Protective effects of taurine on endotelial cells impaired by high glucose and oxidized low density lipoproteins. Eur J Nutr 2007; 46: 431-438. [ Links ]
49. Rasouli ML, Nasir K, Blumenthal RS, Park R, Aziz DC, Budoff MJ. Plasma homocysteine predicts progression of atherosclerosis. Atherosclerosis 2005; 181 (1): 159-165. [ Links ]
50. Ahn CS. Effect of Taurine Supplementation on Plasma Homocysteine Levels of the Middle-Aged Korean Women. Adv Exp Med Biol 2009; 643: 415-422. [ Links ]
51. Franconi F, Bennardini F, Mattana A, Miceli M, Ciuti M, Mian M, Gironi A, Bartolomei G, Anichini R and Seghieri G. Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: Effects of taurine supplemmentation. Am J Clin Nutr 1995; 61 (5): 1115-1119. [ Links ]
52. Spohr C, Brøns C, Winther K, Dyerberg J, Vaag A. No effect of taurine on platelet aggregation in men with a predisposition to type 2 diabetes mellitus. Platelets 2005; 16 (5): 301-305. [ Links ]
53. Wójcik OP et al. The potential protective effects of taurine on coronary heart disease. Atherosclerosis 2010; 208 (1): 19-25. [ Links ]
54. Liu L, Liu I, Ding J et al. Ethnic and environmental differences in various markers of dietary intake and blood pressure among Chinese Han and three other minority peoples of China: results from the WHO cardiovascular disease and alimentary comparison (CARDIAC) study. Hypertens Res 2001; 24 (3): 315-322. [ Links ]
55. Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation 1987; 75 (3): 525-532. [ Links ]
56. Hayes KC, Pronczuck A, Addesa AE, Stephan ZF. Taurine modulates platelet aggregation in cats and humans. Am J Clin Nutr 1989; 49: 1211-1216. [ Links ]
57. Ricci C, Pastukh V, Leonard J, Turrens J, Wilson G, Schaffer G, and Shaffer SW. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol Cell Physiol 2008; 29 (2): 413-422. [ Links ]
![]() Correspondence:
Correspondence:
Cristina de la Puerta.
Hospital Ramón y Cajal.
Ctra. de Colmenar, km 9,100.
Rua Pedro Gomide, 96, apt.
301, Clélia Bernardes. Madrid.
E-mail: cpuerta@us.es
Recibido: 25-II-2010.
1a Revisión: 18-V-2010.
Aceptado: 21-VII-2010.














