Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.26 no.3 Madrid Mai./Jun. 2011
Glycemic acute changes in type 2 diabetics caused by low and high glycemic index diets
Las variaciones de la glucosa aguda en individuos con diabetes tipo 2 causada por las dietas de bajo y alto índice glucémico
C. E. Gonçalves Reis1 and J. Dullius2
1College of Health Sciences. Universidade de Brasilia. Brazil. 2College of Physical Education. Universidade de Brasilia. Brazil.
ABSTRACT
Introduction: Low-glycemic index diets may improve the glycemic control in type 2 diabetes but the debate over their effectiveness continues.
Objectives: To test the effects of low-glycemic index diets on acute glycemic control (2 days) by measuring capillary blood glucose in patients with type 2 diabetes.
Methods: This was a crossover randomized clinical trial with 12 type 2 diabetics which were randomly divided into 2 groups and targeted the following draft diets for low and high glycemic index (LGI and HGI) for 2 consecutive days in 2 consecutive weeks. Group 1 followed an LGI diet in week 1 and an HGI diet in week 2, group 2 adopted the contrary. They were oriented to maintain medication and lifestyle and to follow the recommendations. Measurements were made of glycemia capillaries in 2 days (fasting, before lunch, post-prandial lunch and before dinner) and one last in fasting on day 3. A food record during the days and the counting of carbohydrates meals was made. The software SigmaStat (version 2.03) was used, with a statistical significance criterion of p < 0.05.
Results and discussion: The amount of carbohydrates ingested by the LGI group was lower (p < 0.01), showing that the adoption of this diet reduces the intake of carbohydrates, being favorable for diabetics. Mean blood glucose on the first day was lower in the LGI group (p < 0.05).
Key words: Diabetes mellitus. Glycemic index. Blood glucose. Carbohydrate.
RESUMEN
Introducción: Dietas de bajo índice glucémico pueden mejorar el control glucémico en la diabetes tipo 2, pero sigue el debate sobre su eficacia.
Objetivos: Evaluar los efectos de las dietas bajas en el índice glucémico en el control glucémico agudo (2 días) por la medición de glucosa en sangre capilar en pacientes con diabetes tipo 2.
Métodos: Se realizó un ensayo clínico cruzado y aleatorizado con 12 pacientes diabéticos tipo 2. Fueron divididos en 2 grupos: dietas de bajo y alto índice glucémico (BIG y AIG). Las dietas fueron consumidas por 2 días consecutivos, en 2 semanas distintas. Para el grupo 1 fue administrado la dieta BIG en la semana 1 seguida de la dieta AIG en la semana 2. En contrario se dio para el grupo 2. Se recomendó a los pacientes que mantuviesen la medicación y el estilo de vida estables. Se midió y registro la glucemia capilar en 2 días (en ayunas, antes y después (2 h) del almuerzo y antes de la cena) y en ayuno del día 3. Durante los días del estudio, se hicieron un registro de los alimentos y el conteo de carbohidratos. Para el tratamiento estadístico (p < 0,05) se utilizó el programa SigmaStat (versión 2.03)
Resultados y discusión: La ingesta de carbohidratos fue menor (p < 0,01) en el BIG, sugiriendo que la utilización de esta dieta reduce la ingesta de carbohidratos, siendo favorable para los diabéticos. La media de glucosa en sangre en el primer día fue inferior en el grupo BIG (p < 0,05).
Palabras clave: Diabetes mellitus. Índice glucémico. Glucemia. Hidrato de carbono.
Abbreviations
BMI: Body Mass Index.
CHO: Carbohydrates.
DM: Diabetes Mellitus.
GI: Glycemic Index.
GL: Glycemic Load.
HGI: High Glycemic Index.
LGI: Low Glycemic Index.
SMBG: Self-monitoring of Blood Glucose.
T2DM: Type 2 Diabetes Mellitus.
Introduction
Type 2 Diabetes Mellitus (T2DM) is a disease resulting mainly from the dysfunction in the carbohydrate metabolism, characterized by hyperglycemia.1 Its prevalence in recent decades has been increasing, reaching nearly 6% of the population,2 data shows that in the world there are about 246 million people with diabetes3 and this number is expected to grow to 366 million in 2030.4 The importance of glycemic control in the prevention of chronic complications of DM was demonstrated by the United Kingdom Prospective Diabetes Study5, as well as the self-monitoring of blood glucose (SMBG) role in the control of diabetes mellitus and its substantial importance in assessing a diabetic type 2.6,7
Carbohydrates (CHO) are one of the factors that most influence glycemic control. According to the American Diabetes Association both the quantity and quality of CHO should be observed in a diabetic's diet.8 The quality of the CHO is reflected by its glycemic index and the quantity by counting the amount of CHO. Jenkins et al., (1981) proposed the term glycemic index (GI), which led to classify CHO according to their speed of glycemic responses.9 The Glycemic Load (GL) is a reference that takes into account the GI and the amount of CHO consumed and tends to reflect the effect of this on blood glucose.10 The CHO counting value as a tool to aid in the control of blood glucose levels is already set. Carbohydrate counting is a method that has been recommended as a tool to be used to determine the amount of CHO to be consumed at each meal and assist in glycemic control.11
The regular consumption of foods with a high glycemic index (HGI) has been associated as a risk factor for diseases such as diabetes, obesity and cardiovascular disease and a low glycemic index (LGI) as preventive to such diseases and recommended in their treatment. LGI diets in general have a higher amount of fiber, helping to reduce the absorption of dietary cholesterol, contributing to a greater release of satiety signals, such as prolonged recurrence of hunger, thus helping in weight control. In addition, are shown in diabetic patients lower glycemic response immediately and, consequently, a lower insulin discharge, helping to keep blood glucose levels less oscilant.12-15 Among the several interferences in glucose oscillations in a diabetic subject, the diet is essential because it generates a direct change in blood glucose levels. Thus, it is important to help the patient understand the factors that interfere with their food selection and then make appropriate choices.16-17
Research on GI diets with humans has been largely developed, but investigations about acute blood glucose oscillations are few. This study aims to evaluate acute glycemic control (2 days) by measuring intensive capillary blood glucose due to the adoption of dietary advice to follow LGI and HGI diets in the dietary patterns of type 2 diabetics and correlating the amount of CHO consumed at lunch with their glycemic response too.
Methodology
Study design and sample selection
This is a crossover randomized clinical trial. The study population consisted of type 2 diabetic participants in a program of diabetes education and health from the University of Brasilia, Brazil. The selected sample consisted of 22 individuals. The calculation of sample size for the current study was established considering blood glucose as the main variable. Was adopted a statistical power of 80% and a significance level of 5%. The inclusion criteria were a diagnosis of type 2 diabetes,18 be literate, age between 40-75, demonstrate competence to perform SMBG, have followed the guidelines of the researchers about the GI diets and signed the written informed consent. The study excluded candidates who had eating and/or other endocrine disorders, a history of myocardial infarction, cancer, smoking and alcoholism, liver disease, pregnant women, athletes, people with renal failure and chronic obstructive pulmonary disease.
Experimental design of the study
After selecting the sample, there was an initial meeting with all participants to explain the purpose of the research, how to follow it and the importance of complying with all the directives from the team. We applied an initial questionnaire to collect clinical and epidemiological data, and gave guidelines. Then, the sample was divided randomly into two groups (LGI and HGI) in the next two days and on the morning of the third day, Group 1 was instructed to keep the LGI diet and group 2 the HGI diet. All volunteers received a follow-up form, and were explained in detail all the procedures to be followed by the participants. On the fourth day there was another meeting with the team to deliver the forms, review and verify the food records for 2 days. The following week, the methodology was repeated, but with the reversal of the groups: Group 1 on HGI diets and Group 2 on LGI diets, with the final re-evaluations too.
The variables collected included: demographics-gender, date of birth, time of diagnosis of diabetes, the use of hypoglycemic medications and insulin, schooling. This form contained explanations about the research, contact phone numbers in case of questions, recommendations, the type of diet to follow (LGI or HGI), days to follow, examples of foods to give preference to and which to avoid eating during the study according to the GI diet to be ingested.
For the collection, the volunteers had during the days of experiment a particular form with specifically boxes to put the values of blood glucose measurements, medicine used, the food record and their schedules. Participants were instructed to keep stable medication and lifestyle, not to practice intense physical activities and to strictly follow the recommendations of the team during the period, especially during the days of data collection. Figure 1 illustrates the experimental design of the study.
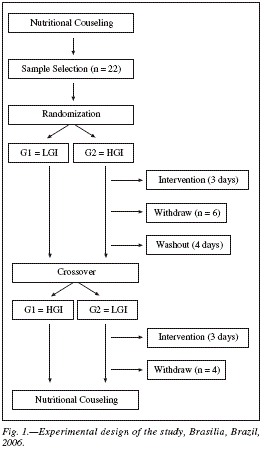
All volunteers who met the selection criteria had access to all the evaluations completed at the end of the study and received nutritional counseling for two months. The protocol of this study was approved (n. 0021.0.012.000-03) by the Ethics Committee in Human Research of the School of Health Sciences at the University of Brasilia, Brazil. All volunteers were informed about the objectives of the study and signed the written informed consent.
Assessment of Glycemic Response
Post-prandial glucose (2 h) was made by finger prick before the start of the study, under the supervision of researchers. After the Kolmogorov-Smirnov test was verified the normality of the glucose data (p = 0.158), demonstrating the homogeneity of the sample.
Glycemic assessment was done with blood collected by finger prick using the One Touch Ultra® glucometer. The volunteers were instructed and trained to take their own glucose measurements during the two days of intervention -fasting, pre-lunch, post-prandial (2 hours after lunch), pre-dinner- and a final fasting measurement on the third day. To do this, all individuals were provided with enough and extra test strips to being used for their own glucose measurements. The results were collected and recorded by the individuals in specific places in the particular forms, with their schedules.
Dietary counseling
At the beginning of the study was done dietary advice to the adoption of high and low GI diets within the usual dietary patterns of individuals. Participants were instructed to choose GI foods corresponding to the experimental group were they are allocated, including in their usual diets during the days of the experiment. For example, the subjects allocated in low-GI group were instructed to consume more fruits, vegetables, salads, legumes, whole grains, dairy products. To facilitate such a procedure a table of CHO counting and glycemic index foods was developed and provided with the breakdown of foods according to their GI so that they can better include the food according to the type of diet to follow. The food selection on this table was made according to the International Table of GI values.19 At the end of the study were made analysis of food records to check if the instructions were followed.
Anthropometric measurements
The height of the volunteers was measured using a stadiometer, scale of 0 to 220 cm, with an accuracy of 0.1 cm (SECA Model 206®), fixed to the wall. The weight was measured using an electronic balance with a 150 kg capacity and 50g increments (Toledo Brazil, Model 2096® PP), with individuals in the standing position without shoes and wearing light clothing.20 The Body Mass Index (BMI) was calculated relating the weight (kg) and height (m2) (Bray and Gray, 1988)21 and classified according to World Health Organization criteria (WHO, 1995).22
Assessment of Food Intake
Before the study, all volunteers were trained to record their food intake on collection days. The food records were recorded on appropriate forms, specifying the time of the meal, food type, preparation and quantity of portion sizes. Each food record was reviewed in the presence of the volunteer in order to ensure their accuracy. For the CHO calculation in each meal, the conversion of portion sizes to grams was done using a table for the evaluation of food consumption in portion sizes,23 and to verify the amount of CHO in the meal Brazilian Table of Food Compo-sition24 was used, obtained by the sum of the amount of each food.
Statistical Analysis
The Kolmogorov-Smirnov test was applied to verify the normality of the variables. To evaluate the differences between glucose levels and CHO intake resulting from the LGI and HGI diets the Wilcoxon test and Paired t-test were used. To correlate the amount of CHO intake at lunch with their respective postprandial glycemia the Spearman's rank correlation coefficient was used and to compare the glycemic values between the days of each diet the Kruskal-Wallis test was used. The analyses were performed using the software SigmaStat (version 2.03), with a statistical significance criterion p < 0.05 (95% confidence). The results of sample characterization and glycemic responses and food intake are presented as mean ± standard deviation.
Results
Three subjects from initial sample refused to participate in the second stage, three had incomplete filled forms and four had personal problems, thus their data was not used. Data of twelve individuals was used for the analyses. Examples of situations that were reported by excluded volunteers: Subject 1: "I woke up at dawn to take my grandson to the hospital, he ended up hospitalized. I spent all night worried about the situation"; Subject 4: "I did not do physical activity on these two days for medical reasons, because I was doing a treatment for varicose veins"; Subject 7: "I did not follow the diet because there was a party at my work and I ate far beyond the usual", in addition to health problems reported by subject 11: flu, diarrhea and malaise (table I).
After the count of total carbohydrate ingested in the days of the experiments, was calculate the means ± standard deviation. There was a significant increase in carbohydrate intake in high-GI group (238 ± 71.5) compared to the low-GI group (176.7 ± 56.2) (p < 0, 01). Were done means ± standard deviation of the number of meals per day of experiment for each treatment (AIG = 5.0 ± 1.4; BIG = 5.3 ± 1.4) (p = 0.42). These results show that although the IG does not influence the number of meals per day, leads to an increase in the total carbohydrate in the diet.
The total amount of CHO ingested by the LGI group per meal (43.6 ± 25.2 g) was lower than the HGI group (61.1 ± 36.2 g) (p < 0.01). When compared for days, on the first day the LGI group ingested 43.7 ± 23.1 g per meal while the HGI group 65.0 ± 40.0 g (p < 0.01) and the second day the LGI group consumed 43.5 ± 27.4 g per meal against 57.5 ± 31.7 g HGI group (p < 0.01). Figure 2 shows the mean ± standard error of amount of carbohydrates ingested per meal in the everyday diet.
On the first day the mean blood glucose was higher in the HGI group (148 ± 62 mg/dl) compared with the LGI group (127 ± 30 mg/dl) (p < 0.05). By the second day, fasting glucose levels had the same average value (132 mg/dl) and no significant difference in the total daily average (p = 0.78). In the comparison of fasting on the third day there was also no statistical difference (p = 0.12). In the evaluation of all glucose measurements, the average blood glucose levels were not different between the two diets (p = 0.37). Figure 3 shows the average of every day blood glucose levels. There were no significant differences comparing blood glucose levels between the days of each diet: LGI diet (p = 0.79) and HGI diet (p = 0.52), showing no improvement or worsening of glycemic control over the days of the research.
Figure 4 shows the correlation between the amount of CHO consumed at lunch with their respective postprandial glycemia (2 h).
Discussion
Currently one of the discussions regarding the dietary treatment of T2DM is about the type of CHO. It is closely related to glycemic changes, which could lead to benefits and improvements in the metabolic parameters of the patients.25 According to the recommendations of American Diabetes Association (ADA),8 nutritional therapy is extremely important in the prevention and treatment of T2DM, with the objective of control blood glucose levels, normalize blood pressure values, avoid gaining weight and the complications of metabolic disorder, as well as other objectives. The recommendations for the quantity and quality of CHO should be made, always seeking the general benefits of using the glycemic index and glycemic load.
At the beginning of the study there was great interest from individuals to participate in the research because they should do several SMBG per day and understand how their body responds to certain types of food. The volunteers were encouraged to give more attention to the foods they were consuming, understand better how the changes in consumption affect blood glucose and what factors are associated with these oscillations, such as intrinsic to foods and environmental factors.
On the report of the HGI days, there was a description of the foods that were included in the diet, e.g. chocolate cake, banana and guava candy, soda, pudding, candy in general, fritters, macaroni, chocolate, condensed milk, and foods that often the health professionals who are monitoring the patients ultimately restrict or even prohibit. The ban comes in response to the fact that these foods are HGI, since they may adversely affect the glycemic control in diabetics. However, in the present study, this result was not so evident.
As reported by Brand-Miller et al.,25 Ludwig,26 Sartorelli & Cardoso27 LGI diets are used for diabetics to improving the glycemic profile, but in our study we were able to perceive this benefit only on the first day of intervention. It was noted that after the end of the first day, there was a balance of blood glucose levels, and the mean fasting values of the second day were the same (132 mg/dl), regardless of the diet followed. It may be because the HGI group proved to be a bit more concerned about glucose levels, due to the high values of the previous day, which led to an improvement in dietary habits and glycemic control.
A greater focus on nutrition education is needed, showing that diet control is necessary in the glycemic control.28 Nutritional education is highlighted in epidemiological studies where the results point to a correlation between eating behavior and disease. It is the part of nutrition science that applies to directing its resources toward learning, adapting and the acceptance of healthy eating habits in line with promoting the health of the individual and the community.29,30 Monitoring through a diabetes education program is extremely important, with an emphasis on diet therapy, physical activity and self-care.16
When the quantity of CHO ingested at lunch with their respective blood glucose levels were correlated, a weak correlation was found between the variables (p = 0.12), showing that despite the average CHO ingested in the meals had been greater in the HGI diets (p < 0.01), no great impact was shown on the glycemic response. This is evidence that a HGI diet is associated with a greater consumption of CHO, but not necessarily to a loss of glycemic control. In this study, the amount of CHO is not linked to blood glucose, as can be seen in the results of the CHO ingestion at lunch with postprandial glucose.
Although recent studies show LGI diets are beneficial in the treatment of T2DM,31,32 other results are still conflicting.33 In practice it was possible to observe significant changes in acute glucose due to the adoption of LGI diets only on the first day, this may be because of the difficulties encountered by individuals following the diet in their daily lives, and that psychosocial factors influencing their decisions regarding food choices. The group showed the adoption of healthy choices when blood glucose levels were altered, reaching a point of knowledge desirable in a work of nutrition education, a fact corroborated by the study of Miller et al.34
Conclusion
In our study the amount of CHO ingested per meal by the LGI group (43.6 ± 25.2 g) was lower than the HGI group (61.1 ± 36.2 g) (p < 0.01), showing that the adoption of the LGI diet reduces the CHO intake, being favorable for people with diabetes. Although is know the benefits of low-GI diets on glycemic and metabolic control in type 2 diabetics, in our study there was significant difference in acute glycemic control due to the adoption of low-GI diet only on the first day (p < 0.05).
The results of our study are important because they show that the nutritional instructions for adoption of low-GI diets in free-living condition is valid, providing a lower intake of carbohydrates, contributing to a lower energy intake and improvement in glycemic control. It is necessary to conduct well-controlled clinical studies evaluating the effect of glycemic index on acute glycemic control in patients with diabetes, to show the effectiveness of the nutritional tool.
Acknowledgements
Team Doce DESAFIO/UnB, One Touch Ultra Johnson & Johnson Medical, National Council for Scientific and Technological Development, Brazil; Unit of Extension at the University of Brasilia, Wesley de Jesus Silva for statistical analysis.
References
1. Sociedade Brasileira de Diabetes. Atualização Brasileira sobre Diabetes. Rio de Janeiro: Diagraphic; 2006. [ Links ]
2. Ministério da Saude, Organização Pan-americana de Saúde. A vigilância, o controle e a prevenção das doenças crônicas não transmissíveis: DCNT no contexto do sistema único de saúde Brasileiro. Brasília: MS; 2005. [ Links ]
3. International Diabetes Federation. Diabetes epidemic out of control. Cape Town, 2006. (cited 2006 Dec 4). Available from: <http://www.idf.org/home/mdex.cftn?unode=7F22F450-B1ED-43BB-A57C-B975D16A812D> [ Links ].
4. Wild S, Roglic G, Green A, Sicree R, King H. Global Prevalence of Diabetes - Estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27 (5): 1047-1053. [ Links ]
5. United Kingdom Prospective Diabetes Study Group. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405-412. [ Links ]
6. Maia FFR, Araújo LR. Impacto do sistema de monitorização continua da glicose em indivíduos diabéticos. Rev Assoc Med Bras 2006; 52 (6): 395-400. [ Links ]
7. Oliveira CHMC, Berger K, Souza SCAL, Marui S, Khawali C, Hanache OM et al. Monitorização Contínua de Glicose: Análise Crítica Baseada em Experiência ao Longo de Um Ano. Arq Bras Endocrinol Metab 2005; 49 (6): 983-990. [ Links ]
8. American Diabetes Association . Nutrition Recommendations and Interventions for Diabetes: A position statement of the American Diabetes Association. Diabetes Care 2008; 31 (Suppl. 1): S61-78S. [ Links ]
9. Jenkins DJA, Wolever TMS, Taylor RH, Barker H, Fielden H, Baldwin JM et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981; 34 (3):362-66. [ Links ]
10. Brand-Miller JC, Foster-Powell K, Colagiuri S. A nova revolução da glicose. Rio de Janeiro: Elsevier; 2003. [ Links ]
11. Sociedade Brasileira de Diabetes. Manual Oficial de Contagem de Carboidratos. São Paulo: SBD; 2003. [ Links ]
12. Jenkins DJA, Kendall CWC, Augustin LSA, Francesch Si, Hamidi M, Marchie A et al. Glycemic index: overview of implications in health and disease. Am J Clin Nutr 2002; 76(Suppl. 1): 266S-73S. [ Links ]
13. Colombani PC. Glycemic index and load - dynamic dietary guidelines in the context of disease. Physiol Behav 2004; 83(4): 603-10. [ Links ]
14. Sunyer FXP. Glycemic index and disease. Am J Clin Nutr 2002;76 (Suppl. 1): 290S-8S. [ Links ]
15. Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr 2002; 76 (Suppl. 1):274S-80S. [ Links ]
16. Dullius J, Borges ED. PROAFIDI/UnB: Educação em Diabetes por meio de Programa Orientado de Atividades Fisicas. Diabetes Clinica 2004; 5: 355-364. [ Links ]
17. Nascimento MAB. Avaliação de uma proposta de educação nutricional para portadores de diabetes mellitus tipo 2 [Mestrado]. Brasília: Universidade de Brasília; 2003. [ Links ]
18. American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2006; 29 (Suppl. 1): S43-S48. [ Links ]
19. Foster-Powell K, Holt SHA, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002; 76 (1): 5-56. [ Links ]
20. Jelliffe DB. Evolución del estado de nutrición de la comunidad. Ginebra, Organización Mundial de la Salud 1968. [ Links ]
21. Bray GA, Gray DS. Obesity I: Phathogenesis. Western J Med 1988; 149 (4): 429-441. [ Links ]
22. World Health Organization. Physical Status: The use and interpretation of antropometry. Geneva; 1995 (WHO Technical Report Series 854) 452p. [ Links ]
23. Pinheiro ABV, Lacerda EMA, Benzecry EH, Gomes MCS, Costa VM. Tabela para avaliação de consumo alimentar em medidas caseiras. Rio de Janeiro; 1994. [ Links ]
24. Núcleo de Estudos e Pesquisas em Alimentação. Tabela brasileira de composição de alimentos / NEPA-UNICAMP.- Versão II, 2o ed., Campinas, SP: NEPA-UNICAMP, 2006. [ Links ]
25. Brand-Miller JC, Hayne S, Etocz P, Colagiui S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003; 26 (8): 2261-7. [ Links ]
26. Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002; 287 (18): 2414-23. [ Links ]
27. Sartorelli DS, Cardoso MA. Associação entre carboidratos da dieta habitual e diabetes mellitus tipo 2: evidências epidemiológicas. Arq Bras Endocrinol Metab 2006; 50 (3): 415-426. [ Links ]
28. Geraldo JM, Alfenas RCG, Alves RDM, Salles VF, Queiroz VMV, Bittencourt MCB. Intervenção nutricional sobre medidas antropométricas e glicemia de jejum de pacientes diabéticos. Rev Nutr 2008; 21: 329-340. [ Links ]
29. Sartorelli DS, Franco LJ, Cardoso MA. Intervenção nutricional e prevenção primária do diabetes mellitus tipo 2: uma revisão sistemática. Cad Saúde Pública 2006; 22 (1): 7-18. [ Links ]
30. Cervato AM, Derntl AM, Latorre MRDO, Marucci MFN. Educação nutricional para adultos e idosos: uma experiência positiva em Universidade Aberta para a Terceira Idade. Rev Nutr 2005; 18 (1): 41-52. [ Links ]
31. Opperman AM, Venter CS, Oosthuizen W, Thompson RL, Vorster HH. Meta-analysis of the health effects of using the glycaemic index in meal-planning. Br J Nutr 2004; 92: 367-381. [ Links ]
32. Venn BJ, Green TJ. Glycemic index and glycemic load: measurement issues and their effect on diet-disease relationships. Eur J Clin Nutr 2007; 61 (Suppl. 1): S122-31. [ Links ]
33. Wolever TM, Gibbs AL, Mehling C, Chiasson JL, Connelly PW, Josse RG et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr 2008; 87 (1): 114-25. [ Links ]
34. Miller CK, Gutshcall MD, Mitchell DC. Change in food choices following a glycemic load intervention in adults with type 2 diabetes. J Am Diet Assoc 2009; 109 (2): 319-24. [ Links ]
![]() Correspondence:
Correspondence:
Caio Eduardo G. Reis.
QI 04 Bloco E apto. 108 - Guará 1.
P.O. BOX: 71010-052 DF, Brazil.
E-mail: caioedureis@gmail.com
Recibido: 7-I-2010.
1a Revisión: 25-III-2010.
Aceptado: 10-X-2010


















