My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.26 n.4 Madrid Jul./Aug. 2011
Behavioral analysis of Wistar rats fed with a flaxseed based diet added to an environmental enrichment
Análisis del comportamiento de ratas Wistar alimentadas con una dieta a base de linaza añadidos a un enriquecimiento ambiental
J. Azevedo de Meneses1, C. A. Junqueira Lopes1, L. G. Coca Velarde2 and G. Teles Boaventura1
1Department of Nutrition and Dietary. Fluminense Federal University. Brazil.
2Department of Statistical. Fluminense Federal University. Brazil.
ABSTRACT
Flaxseed has a high content of n-3 fatty acids and its intake associated with an environmental enrichment may promote distinct behavioral results upon habituation and animal behavior. This work aimed to evaluating animal behavior under the use of these two tools in the Open Field Test. Thirty-six male Wistar rats were divided into 6 groups (n = 6): FEEG, receiving chow made up of flaxseed and kept in enriched environment; FSEG, receiving flaxseed based diet and kept in a standard environment; CEEG, receiving casein based diet and kept in enriched environment; CSEG, receiving casein based chow and kept in standard environment; MCEEG, receiving chow made up of casein but modified so as to provide the same content of fibers and lipids found in flaxseed diet and kept in enriched environment; MCSEG, receiving modified casein based diet and kept in standard environment. All animals were kept under controlled temperature, collective cages and dark/light cycle, receiving chow and water ad libitum, except for MCEEG and MCSEG, which were pair fed with FEEG and FSEG, respectively. Chow intake and animal body weight were evaluated twice in a week. Animals were maintained in these groups from the first until the second month of life, by the time when 3 day tests in Open Field Test began. Finishing the tests, animals were sacrificed and their brains were obtained in order to calculate the relative brain weight. Our results show an interplay between flaxseed and environmental enrichment in habituation to a new environment, making the animals more manageable and less stressed.
Key words: Flaxseed. Environmental enrichment. Open field test. Rat. Behavioral.
RESUMEN
La linaza posee una gran cantidad de ácidos grasos n-3 y su consumo asociado a ambiente enriquecido, puede promover diferentes resultados comportamentales sobre el animal y su habituación. Este trabajo tubo por objetivo evaluar el comportamiento animal utilizando dos herramientas en el Open Field Test. Treinta y seis ratón Wistar fueron divididos en 6 grupos (n=6): FEEG, que recibió dieta a base de linaza y fué mantenido en ambiente enriquecido; FSEG, que recibió dieta a base de linaza y fué mantenido en ambiente padrón; CEEG, que recibió dieta a base de caseína y fué mantenido en ambiente enriquecido; CSEG, que recibió dieta a base de caseína y fué mantenido en ambiente padrón; MCEEG, que recibió dieta a base de caseína con modificaciones de modo a proporcionar el mismo contenido de fibras y grasa encontrados en la dieta a base de linaza, y mantenido en ambiente enriquecido; MCSEG, que recibió dieta a base de caseína modificada y fué mantenido en ambiente padrón. Todos los animales tubieron temperatura ambiente controlada, jaulas colectivas (n = 3) y ciclo claro/oscuro (12 h), recibiendo água y ración ad libitum, excepto los grupos MCEEG y MCSEG que fueron sometidos a sistema pair feeding con los grupos FEEG y FSEG, respectivamente. El consumo y peso corporal de los animales fué medido dos veces por semana. Los animales fueron mantenidos en sus respectivos grupos a partir del primer mes de vida y hasta el segundo, cuando se inició un período de pruebas en el Open Field Test. Al término de las pruebas se sacrificaron los animales y se retiraron sus cerebros para calcular el peso relativo. Nuestros resultados muestran una interacción entre la linaza y el enriquecimiento ambiental en la habituación a un nuevo ambiente, haciendo que los animales sean mas manipulables y menos nerviosos.
Palabras clave: DHA. Enriquecimiento ambiental. Openf Field test. Ratón. Comportamiento.
Introduction
Currently, environmental enrichment is a very common means of improving animal well-being, especially for laboratory animals. Although environmental enrichment seems to be a possible way for improving the well- being of animals, the consideration of housing laboratory animals should not only focus solely on animals well-being, manpower and economics but also on the precision and accuracy of the experimental results.1
Environmental Enrichment (EE) has been drawing attention of many studies on the grounds that besides it can be used as a tool for recuperation of lesions and cerebral diseases,2 it can also imitate natural habitat of experimental animals, causing improvement in welfare under laboratorial environment.3,4 Countless studies have shown its effects under behavior development and cognitive skills acquisition.5 In fact, precocious interventions can affect sociability, learning, physical development and neurogenesis in some species of rodents.6 For that reason, researches on environmental enrichment have often focused in investigating the impacts of different environmental conditions of breeding upon behavioral organization and/or nervous system of studied animals.7,8
The Canadian psychologist Donald O. Hebb was the first researcher to be interested in environmental enrichment impact upon behavior in the last century. He discovered that animals bred in large environment and with wide range of objects and spatial configurations presented more superior learning skills than animals bred in laboratories in smaller and not enriched environments.7 Environmental enrichment produces effects that go beyond behavioral/physiological outcomes; it offers responses in cerebral plasticity, which varies from biochemical parameters to dendritic trees, gliogenesis, neurogenesis and finally improvement of learning and memory.2
Docosahexaenoic acid (DHA) is known for its effects upon cerebral function, humor and behavior. It acts as one of the "building blocks" of cerebral growth and development-cell membranes of brain are highly enriched with DHA9. This fatty acid is incorporated in high amount into structural lipids during central nervous system development, being a deficient accumulation related with behavioral abnormalities.9,10,11,12,13. Furthermore, many animal studies show that this acid improves learning, visual processes, memory and concentration.9,11,13 Consequently, it is not controversial that DHA can affect cerebral and behavioral functions and that its intake leads to fetal development.14,15
Flaxseed also contributes to behavioral response as it contains high content of alpha linolenic acid (C18: 3n-3, ALA), which is a long chain polyunsaturated fatty acid (LC-PUFA), essential, from n-3 series, that is converted to eicosapentaenoic acid (C20:5n-3, EPA) and docosahexaenoic acid (C22:6n-3, DHA), two compound known by their benefits upon cardiac health, arthritis, thrombotic diseases and, especially, cerebral functions.16
A powerful tool to measure behavior is the Open Field Test. Since its introduction, 80 years later, it has reached the status of one of the most used instrument in animal psychology. This popularity stems from its simplicity and agility to measure behavior and wide applicability, which is generally accepted as interpretation.17
Considering that not only enrichment environmental but also flaxseed exerts related functions upon cerebral physiology, especially in habituation and behavior, this study aimed at evaluating the behavior of rats fed with flaxseed in enrichment environmental using the Open Field Test.
Material and methods
Animals
Thirty-six males Rattus novergicus were used in the biological assay, albinus variety, Rodentia mammalia, Wistar strain, offspring (F1), stemmed from Experimental Nutrition laboratory (LABNE), males, offspring (F1), stemmed from Experimental Nutrition Laboratory (LABNE) from Nutrition and dietetic department of Nutrition College at Fluminense Federal University, Niteroi, RJ, Brazil. Animals came from other generation (F0), fed with the respective chow at the moment of the monogamic match. The protocol of this experiment was approved by the Ethics Committee in Research from Federal Fluminense University (UFF). All procedures were carried out in accordance with the norms from Brazilian College of Animal Experimentation (COBEA).
Experimental design
Pups were divided into three groups (n = 12) according to the chow received by F0: Flaxseed group (FG), receiving chow made up of casein with 25% of flaxseed, Control group (CG), receiving casein based chow, Modified Control group (MCG), receiving chow made up of casein added to 4% of fibers and 2% of soy oil, aiming at reaching the same amount of these nutrients in flaxseed chow. These dietetic groups were divided into 6 groups: FG with EE (FEEG); FG without EE (FSEG); CG with EE (CEEG); CG without EE (CSEG); MCG with EE (MCEEG) and MCG without EE (MCSEG). Animals received diet and water ad libitum, except for MCEEG and MCSEG, which were maintained in a pair feeding scheme with FEEG and FSEG, respectively. Chow intake and animal body weight were evaluated twice in a week throughout the experiment. All animals were kept under controlled temperature (22oC), and dark/light cycle (12/12 h).
Environmental enrichment
Animals subjected to this process were maintained 24 hours per day in a propylene cage with total dimensions of 25.5 cm, 33.5 cm and 40.5 cm (height, width and profundity, respectively), with a unit of each object: metal wheel for exercise with 12 cm diameter, plastic shelter with dimensions of 9.5 cm, 14.2 cm and 10.2 cm (height, width and profundity, respectively), metal seesaw with dimensions of 4.5 cm, 6.0 cm and 26.0 cm (height, width and profundity, respectively), plastic cubes with dimensions of 5.0 cm edge and rubber balls with 6.5 cm diameter. Such objects were alternated at random twice per week. Animals without enriched environment were kept in similar cages without these objects. Three animals were placed in each cage.
Behavioral analysis
Open-field test was used for behavioral analysis, in which a square arena with 33.0 cm of height and 80.5 cm in each side, divided into 16 quarters. The analyses were performed at the end of the period of enriched environment always at the same time, with 24 hours interval, once a Day, during three consecutive days.
Each animal, separately, was allocated at the center of the arena and had its behavior registered for 4 minutes. The behavioral variables were measured according to duration (in seconds) at three degrees of activity18 (Van De Weerd, 1996): high activity: walk and run; low activity: sit down, including small movements of head and feet and inactivity: no movement ("freezing" and "resting"). A software for behavioral register was used "X-Plot-Rat 2005 for Windows" (version 1.1.0 developed by Exploring behavior laboratory from Sao Paulo University, Philosophy college, sciences and arts of Ribeirao Preto, Ribeirao preto, SP, Brazil).
Brain measurement
After the last test, animals received intraperitoneally a lethal dose of Thiopentax (sodium Thiopental) 1 g (DOSEml = 0.15 x animal weight g/100), sedating them and decapitating with a guillotine. Brains were excised and weighted in an analytic scale, Bosch, S 2000 model, with precision of 0.0001 g in order to obtain relative cerebral weight (RCW) that was registered by the division of cerebral weight by body weight and multiplying this result by 100.
Statistical analysis
S-Plus (version 6.0) software was used to analyze all answer variables. For Open-field test results in each Day of the test, Kruskal-Wallis rank sum test was used to test differences among dietetic groups and Exact Wilcoxon rank-sum test to evaluate differences between EEG and SEG. Significance level established for both situations was p < 0.05. For relative brain weight, Kruskal-Wallis rank sum test was used to verify statistical differences with significance level of p < 0.005 for diet and EE.
Results
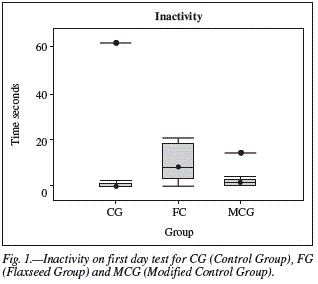
At the first Day of test, diet was the unique factor interfering (p < 0.03) with the duration of animal activity in the Open-field arena, more precisely in the inactivity response variable (fig. 1). FG presented higher inactivity (10 ± 8s), followed by CG (6 ± 18s) and MCG (3 ± 4s).
At the second Day, both diet and EE promoted differences among the groups. The high activity was influenced by diet (p < 0.02), while inactivity was influenced by diet (p < 0.03) and EE (p < 0.01). FG (fig. 2a) was the group with the lowest activity (36 ± 20s), above this was MCG (54 ± 17s), and after the CG (58 ± 19s). Conversely, in inactivity (fig. 2b), FG (19 ± 20s) was superior when compared to CG (8 ± 22s) and MCG (5 ± 10s). EEG was the most inactive (15 ± 19s) (fig. 2c), comparing to SEG (7 ± 17s). When the analysis was concentrated in each dietetic group (fig. 2d), it was perceived that FG was the only one that did not follow the previous result, being FEEG (20 ± 9s) similar to FSEG (17 ± 28s). However, within CG, the CEEG (15 ± 31s) and CSEG (1 ± 1s), and within MCG; MCEEG (9 ± 6s) and MCSEG (2 ± 2s) behaved likewise FG concerning inactivity at the second day of test.
At the third Day of test, the last one, diet was not significant to activities during the test. Nevertheless, EE interfered with low activity more specifically (p < 0.03) and inactivity (p < 0.01). SEG stayed more time sit, making small movements with head and feet (194 ± 12s) in relation to EEG (186 ± 11s) (fig. 3a). The opposite is observed in inactivity, where SEG presented a low duration (4 ± 4), whereas EEG presented more pronounced inactivity (19 ± 7) (fig. 3b). Low physical activity is similar concerning different dietetic groups, CG-CEEG, 182 ± 3; CSEG, 192 ± 5 and FG-FEEG 179 ± 11; FSEG, 196 ± 7, except for MCG, with MCEEG (197 ± 9) surpassing MCSEG (193 ± 20), implying that animals in not enriched environment had bigger values than enriched ones (fig. 3c). However, inactivity follows the same pattern inside the three groups: MCG- MCEEG (9 ± 6) and MCSEG (4 ± 5), CG-CEEG (13 ± 12) and CSEG (3 ± 4), and FG-FEEG (34 ± 19) and FSEG (5 ± 4) (fig. 3d).
As far as cerebral development is concerned, there was influence of the sort of chow consumed (p < 0.05) upon this response variable (table I). Experimental group (FG) obtained the biggest relative cerebral weight (0.63 ± 0.05), followed by MCG (0.56 ± 0.05) and by CG (0.50 ± 0.03). When enriched animals were compared to non-enriched ones, there is a huge numerical difference in the percentage, but not statistically significant, suggesting a better response with environmental enrichment groups.
Discussion
Open-field tests behavioral effects in non-familiar environments, measuring animal emotional activity19. Animals that present low levels of activities (locomotion) in this new environment are classified as more emotive than animals with opposite behavior.17,20 Furthermore, high activity implies environmental exploration, whereas its decrease or opposite activities indicate habituation18. In our study, among the three diets, FG is more familiar to new environments. Analyzing total time of inactivity during the day so as to calculate the percentage in which each group contributed to this finding, it was observed that FG had percentage equal or bigger than 55%, being followed by CG (25-30%) and MCG (maximum of 17%). This information agrees with Hamazaki et al. (1999), who described DHA as a facilitator of habituation. Likewise, Fedorova & Salem (2006) state that habituation makes animals less stressed, a beneficial effect of DHA.
In the same way of DHA, one of the benefits of EE is to accelerate habituation process.21 In our study, enriched animals showed less activity level than animals in standard environment, even considering different dietetic approaches. In two out of three days of test, EE obtained significance when EEG was compared to SEG. It's important to highlight that at the second day despite behaving similar to FEEG, FSEG presented level of inactivity much more similar to CSEG, emphasizing the importance of EE apart from the presence of flaxseed in the diet.
FG showed relative brain weight superior to all other groups due to high concentration of lipids in the cell membranes.10,22 This correlation can be accounted for the fact that a diet rich in polyunsaturated fatty acids may increase the aggregation of fatty acids to brain, reflecting directly in the bigger weight of this organ.
Values resulting from EE did not cause expressive alteration in RCW. However, it was partially expected because although rats submitted to this kind of treatment present higher RCW,23 many studies attribute opposite effects to environmental enrichment.24 Taking this into account, Baumans25 states that the adoption of this protocol needs detailed analysis in a way that its benefits to quality of laboratory animals did not alter experimental data.
Conclusion
Both flaxseed and environmental enrichment proved to be beneficial to quality of animal life. However, EE did not interfered with emotional aspect, made animals more manageable, getting easily used to new situations and causing improvement in welfare under laboratorial environment. Flaxseed produced cooperation of animals, made less stressed animals and resulted in more natural emotional state. On the other hand, our data suggest that animals fed with flaxseed had a better incorporation of essential fatty acids in the brain provided that bigger relative cerebral weight accounted.
References
1. Tsai P-P, Pachowsky U, Stelzer HD et al. Impact of environmental enrichment in mice. 1: Effect of housing conditions on body weight, organ weights and haematology in different strains. Lab Anim 2002; 36: 411-419. [ Links ]
2. Van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci 2000; 1: 191-198. [ Links ]
3. Baumans V. Environmental enrichment for laboratory rodents and rabbits: requirements of rodents, rabbits, and research. ILAR J 2005; 46 (2): 162-170. [ Links ]
4. Balcome JP. Laboratory environments and rodents' behavioural needs: a review. Lab Anim 2006; 40: 217-235. [ Links ]
5. Zimmermann A et al. Enrichment-dependent differences in novelty exploration in rats can be explained by habituation. Behav Brain Res 2001; 121: 11-20. [ Links ]
6. Escorihuela RM, Tobeña A. Fernández-Teruel A. Environmental enrichment reverses the detrimental action of early inconsistent stimulation and increases the beneficial effects of postnatal handling on shuttlebox learning in adult rats. Behav and Brain Res 1994; 61: 169-173. [ Links ]
7. Fernández-Teruel A et al. Early-life handling stimulation and environmental enrichment: are some of their effects mediated by similar neural mechanisms? Pharmacol Biochem Be 2002; 73: 233-245. [ Links ]
8. Naka F, Shiga T, Yaguchi M, Okado N. An enriched environment increases noradrenaline concentration in the mouse brain. Brain Res 2002; 9: 124-126. [ Links ]
9. Fedorova I, Salem N Jr. Omega-3 fatty acids and rodent behavior. PLEFA 2006; 75: 271-289. [ Links ]
10. Young C, Martin A. Omega-3 em transtornos de humor: revisão. Rev Bras Psiquiatr 2003; 25 (3): 184-7. [ Links ]
11. Carlson SE. Docosahexaenoic acid and arachidonic acid in infant development. Semin Neonatol 2001; 6: 437-449. [ Links ]
12. Hamazaki T. Administration of Docosahexaenoic Acid Influences Behavior and Plasma Catecolamine Levels at Times of Psychological Stress. Lipids 1999; 34: 33-37. [ Links ]
13. Kalmijn S et al. Polyunsaturated Fatty Acids, Antioxidants, and Cognitive Function in Very Old Men. Am JEpidemiol 1997; 145 (1): 33-41. [ Links ]
14. Simopoulos AP, Bazan NG. Omega-3 Fatty Acids, the Brain and Retina. Basel: Karger, 2009. [ Links ]
15. Silva DRB, Júnior PMF, Soares EA. A importância dos ácidos graxos poliinsaturados de cadeia longa na gestação e lactação. Rev Bras Saúde Matern Infant 2007; 7 (2): 123-133. [ Links ]
16. Tinoco SMB, Sichieri R, Moura AS et al. A importancia dos ácidos graxos trans do leite materno para o desenvolvimento fetal e neonatal. Cade Saúde Pública 2007; 23 (3): 525-534 [ Links ]
17. Walsh RN, Cummins RA. The Open Field Test: A critical review. Psychol Bull 1976; 83 (3): 482-504. [ Links ]
18. Van De Weerd HA. Environmental enrichment for laboratory mice: preferences and consequences. University of Utrecht 1996. [ Links ]
19. Hall CS. Temperament: a survey of animal studies. Psychol Bull 1941; 38: 909-943. [ Links ]
20. Archer J. Tests for emotionality in rats and mice: a review. Anim Behav 1973; 21: 205-235. [ Links ]
21. Van De Weerd HA et al. Effects of environmental enrichment for mice: Variation in experimental results. J Appl Anim Welf Sci 2002; 5: 87-109. [ Links ]
22. Benatti P et al. Polyunsaturated Fatty Acids: Biochemical, Nutritional and Epigenetic Properties. J Am Coll Nutr 2004; 23 (4): 281-302. [ Links ]
23. Rosenzweig MR. Environmental complexity, cerebral change, and behavior. Am Psychol 1966; 21: 321-332. [ Links ]
24. Smith AL, Corrow DJ. Modifications to Husbandry and Housing Conditions of Laboratory Rodents for Improved Well-being. ILAR J 2005; 46 (2): 140-147. [ Links ]
25. Baumans V. Environmental Enrichment for Laboratory Rodents and Rabbits: Requirements of Rodents, Rabbits, and Research. ILAR J 2005; 46 (2): 162-170. [ Links ]
![]() Correspondence:
Correspondence:
Juliana Azevedo de Meneses.
E-mail: jazevedom@yahoo.com.br
Recibido: 21-VI-2010.
Aceptado: 7-XI-2010.