Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.26 no.5 Madrid sep./oct. 2011
Influence of omega-3 fatty acids from the flaxseed (Linum usitatissimum) on the brain development of newborn rats
Influencia de los ácidos grasos omega-3 de la linaza (Linum usitatissimum) en el desarrollo del cerebro de ratas recién nacidas
K. C. Lenzi Almeida1, G. Teles Boaventura2 and Ma A. Guzmán Silva3
1Student of the Post-graduation Program in Pathology. Doctorate in Investigate Pathology. UFF.
2Associate Professor. Department of Nutrition and Dietary. UFF.
3Associate Professor. Department of Pathology. UFF. Niterói. RJ. Brazil.
ABSTRACT
Objectives: The importance of essential fatty acids, in particular the omega-3 family, in the central nervous system development of newborns is well documented. The flaxseed (Linum usitatissimum) is considered one of the best vegetable sources of omega-3 fatty acids. The influence of omega-3 fatty acids from flaxseed on the brain development of newborn rats was evaluated.
Material and methods: Pups of the F1 generation were obtained from 18 female Wistar rats divided in 3 groups (n = 6), FG: fed with diet based on Flaxseed added with casein, CG: Casein, and MCG: Modified Casein supplemented with fibers and soybean oil. Newborn pups were weighted and submitted to euthanasia; brains were collected for evaluation of weight and lipid profile through gaseous chromatography.
Results: Significant increase in brain weight (39%) and relative brain weight (37%) was verified in pups from mothers fed with flaxseed diet. The omega-3 (n-3) fatty acids from the flaxseed were found in abundance in the diet made with this oleaginous and also significant increase in docosahexaenoic acid (DHA) (38%), as well as in total of omega-3 (n-3) fatty acids (62%).
Conclusion: Maternal diet of flaxseed during pregnancy influences the incorporation of omega-3 fatty acid in the composition of brain tissue, assuring a good development of this organ in newborn rats.
Key words: Brain. Essential fatty acids. Flaxseed. Omega-3. Rats.
RESUMEN
Objetivos: La importancia de los ácidos grasos esenciales, en particular la familia omega-3, en el desarrollo del sistema nervioso central de los recién nacidos está bien documentada. La semilla de linaza (Linum usitatissimum) es considerada una de las mejores fuentes vegetales de ácidos grasos omega-3. Se evaluó la influencia de los ácidos grasos omega-3 de la linaza en el desarrollo del cerebro de ratas recién nacidas.
Material y métodos: Las crías de la generación F1 se obtuvieron a partir de 18 ratas Wistar divididas en 3 grupos (n = 6), GL: alimentados con dieta a base de linaza adicionada con caseína, GC: a base de caseína, y GCM: con caseína modificada suplementada con fibras y aceite de soja. Las crías recién nacidas fueron pesadas y sometidas a eutanasia; los cerebros fueron recolectados para la evaluación del peso y el perfil lipídico mediante cromatografía gaseosa.
Resultados: Se verificó aumento significativo en el peso cerebral (39%) y en el peso relativo del cerebro (37%) en las crías de madres alimentadas con la dieta de linaza. Los ácidos grasos omega-3 (n-3) de la linaza se encontraron en abundancia en la dieta elaborada con esta oleaginosa, y también hubo importante aumento en el ácido docosahexaenoico (DHA) (38%), así como en el total de ácidos grasos omega-3 (n-3) (62%).
Conclusión: La dieta materna a base de linaza durante el embarazo influye en la incorporación de ácidos grasos omega-3 en la composición del tejido cerebral, asegurando un buen desarrollo de este órgano en ratas recién nacidas.
Palabras clave: Cerebro. Ácidos grasos esenciales. Semillas de linaza. Omega-3. Ratas.
Abbreviations
EFA: Essential Fatty Acid.
DHA: Docosahexaenoic Acid.
COBEA: Brazilian College of Animal Experimentation.
UFF: Fluminense Federal University.
CG: Casein Group.
FG: Flaxseed Group.
MGC: Modified Casein Group.
ALA: Alpha Linolenic Acid.
AA: Aracdonic Acid.
PUFA: Polyunsaturated Fatty Acid.
LC-PUFA: Long Chain Polyunsaturated Fatty Acid.
ALA: Alpha Linolenic Acid.
Σn-3: Sum of n-3.
UFRJ: Federal University of Rio de Janeiro.
CAPES: Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior.
Introduction
Studies show that the intrauterine1 and postnatal2 nutrition can influence in the risk of occurrence of chronic diseases in the adult, suggesting that precocious nutrition with specific fatty acid has a great effect in more advanced age of life. This demonstrates the importance of an appropriate offer of essential fatty acid (EFA) during gestation, nursing and childhood, constituting vulnerable periods for the brain development.3
Nervous fibers of the brain are involved by an insulating membrane of multiple layers denominated myelin sheath. In a similar way to the insulating of an electric cable, this sheath allows the conduction of the electric pulses along the nervous fiber with speed and precision. When myelin is damaged, the nerves don´t drive the pulses in an appropriate way.4
Between the 7th and 14th post-natal day in rats, during the pick of brain growth and beginning of mielinization, there is a fast accumulation of long chain saturated and unsaturated acids.5 The increment of fatty acid in the brain in development has as source the acids captured from the maternal circulation during the gestation. 6 An appropriate offer of these acids in the prenatal and postnatal period is fundamental for the fetal and neonatal normal development,7,8 as well as, neurological functions.8,9
Myelin sheaths are highly enriched with docosahexaenoic acid (DHA).10 This is incorporated in great amounts in structural lipids during the development of the Nervous System,11 and its deficiency during the development has been related with behavior abnormalities. 12 Flaxseed is a great source of omega-3 fatty acid, direct precursor of DHA, being a functional food of high protein and lipid content; among all the oleaginous the flaxseed has the largest content of this fatty acid.13
The annual world production of this seed is between 2.3-2.5 million tons, being Canada the main producer. In South America, the largest producer is Argentina, with about 80 tons/year. Brazil presents a low production, about 20 tons/year.14
Rats were fed with a flaxseed diet during lifetime and the influence of the omega-3 fatty acid from flaxseed on the development (i.e. body weight, brain weight, brain relative weight) and brain lipid profile was assessed in the rat pups (F1 generation).
Materials and methods
Animals
The experiment was conducted in agreement with the determinations of the Brazilian College of Animal Experimentation (COBEA) and was approved by the Committee of Ethics in Research of the Medicine School/Antônio Pedro University Hospital of the Fluminense Federal University. Pups of the F1 generation were obtained by polygamic mating female Wistar rats, divided in three groups (n = 6): Flaxseed (FG), fed with diet based on flaxseed added with casein, Casein (CG), fed with diet based on casein, and Modified Casein (MCG), fed with diet based on casein supplemented with fibers and soybean oil to reach the macronutrient distribution similar to FG. The respective experimental diet was given to the matrices and their mates from the weaning till the end of the experiment. They were placed in mating for 15 days when they reached sexual maturity, giving the F1 generation after gestation and delivery. Pregnant rats were housed in individual cages, in a temperature controlled (24 ± 2oC) facility with 12-h light-dark cycle. The F1 pups were euthanized 2 hours after birth and used for analysis.
Diets
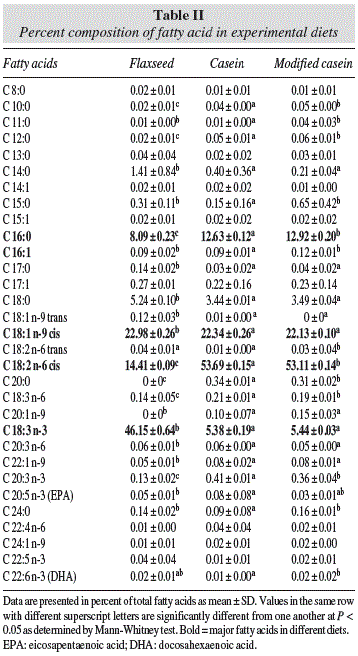
The diets were isoproteic (17% protein) and isocaloric, following the American Institute of Nutrition (AIN-93G) recommendations.15 The composition of each diet is detailed in table I.
Experimental protocol
In each group, females were fertilized by males that received the same diet. FG and CG received food and water ad libitum, while MCG was in pair feeding system with FG, to control differences in food consumption identified in previous studies.
Sample collection
Until two hours after the birth the pups were weight and killed by decapitation. Brains were rapidly removed, weighed, immediately frozen in liquid nitrogen, and stored at -20o C.
Determination of fatty acid composition
Lipid extraction, saponification and methylation of fatty acids in experimental diets and brains were performed in duplicate. Fatty acid methyl esters were quantified by gas-liquid chromatography, by using a Perkin Elmer autosystem XL chromatograph (Perkin Elmer, Norwalk, CT, USA) with an ionizable flame detector and Turbochrom software (Perkin Elmer, Norwalk, CT, USA) as previously described.16
Statistical analysis
Data are presented as the mean and standard deviation. Data of body, brain and relative brain weight were analyzed by ANOVA One Way. Post hoc analysis was performed by Scheffé and Bonferroni tests. Fatty acid composition was analyzed by the nonparametric test of Mann-Whitney. The SPSS software, version 10.0, was used for the analyses and differences were considered significant at P < 0.05.
Results
Fatty acid profile of the experimental diets
A total of 31 fatty acids were found in the experimental diets, flaxseed, casein, and modified casein (table II). In this study, just the acids already described by the literature, as influential, in some way, in the brain development were taken in consideration for analysis and discussion. According to the fatty acids percentage the majority acids in decreasing order for the group FG were the alpha linolenic acid C18:3 (n-3) (ALA) and C18:1 (n-9) cis, proceeded by C18:2 (n-6) cis, while in CG and MCG, they were the fatty acids C18:2 (n-6) cis, C18:1 (n-9) cis and C16:0.
Body, brain and relative brain weight of the pups
Brain weight of the newborn rats from FG were 39% heavier than CG and 25% heavier than MCG (table III), with statistical significance (P = 0.026). There was no statistical difference in the body weight within the diet groups. Relative brain weight showed higher percentage value in FG, with statistical significance (P = 0.0093), when compared to the others diets, since in this group the relative brain weight was 37% higher than CG and 31% higher than MCG. There was no statistical difference among the last two groups.
Fatty acid profile of the pup´s brain
A total of 30 fatty acids were found in the pup´s brain (table IV). According to the fatty acids percentage the majority acids in decreasing order for the group FG were the acid C16:0 and C18:0, proceeded by DHA C22:6 (n-3), while in CG and MCG, they were the same ones, except for the aracdonic acid C20:4 (n-6) (AA). Pup´s brain from FG presented higher content of DHA than the brains from the other diets, being 38% higher than CG and 32% higher than MCG (P = 0.002).
In pup´s brain from FG the omega 3 (n-3) fatty acid sum was 62% higher than CG and 52% higher compared to MCG (table V), being the difference significant (P = 0.002). Similar results were noticed analyzing the polyunsaturated fatty acid (PUFA) sum, since FG was 34% higher compared to CG and 21% higher than MCG (P = 0.002). The essential fatty acid (EFA) sum was also increased in FG being 34% higher than CG and 23% higher compared to MCG, with statistical significance (P = 0.026).
The long chain polyunsaturated fatty acid (LCPUFA) sum in pup´s brain from FG was 2% lower compared to CG and 5% lower than MCG, with statistical difference among the three diets (P = 0.002). Significant decrease (41%) in the omega 6 (n-6) fatty acid sum was noticed in FG compared to the other diets (P = 0.002).
Discussion
The energy supplied by several nutrients in animal species is essential for the maintenance, growth and reproduction. The fat source used in the diet can display significant influence on the growth and use of nutrients in some species. Lipids are the best source of energy used by animals, besides supplying metabolic energy, they are requested for the maintenance of the structure and function of the cellular membrane and supply essential fatty acids to the animals.17
In the current study, the diet based on flaxseed presented a higher alpha lipoic acid (ALA) content when compared to the other diets. Besides this acid, the flaxseed diet presented higher values also in the sum of n-3 fatty acids, PUFA, and EFA. Those differences can be explained by the composition of flaxseed, which presents high percentage (57%) of n-3 fatty acids, causing this increment in the flaxseed diet.18 Similar results had been found with diet containing flaxseed oil, which also presented sufficiently superior concentration of ALA (18:3 n-3), when compared to diets without the oleaginous.19 This result shows that the source of lipids can probably drive the fatty acid profile in the final product, due to differences in the amounts of those acids in the composition of different oils, including also the content of fatty acid of the ω-3 series in which the oleaginous flaxseed is the richest.18
The central nervous system maturation in human begins in the intrauterine phase and persists for seven years, presenting bigger intensity in the first two years of life.20 Already in rats, the beginning also start in the intrauterine phase, however persists only until the thirty day of life.21 The morphogenesis directly associated to the function of the brain requires offer of specific fatty acids, especially of Aracdonic Acid (AA) and DHA. The maternal nutrition becomes very important to this process during gestation and lactation, therefore the embryo and newborn has functional and biochemist increase of the maternal demands of PUFA.12,22,23 PUFA of the series omega-3 are found in the brain and the retina and participate of the growth, contributing for the process of mielinization, and development of the vision function, in the psychomotor development, and some aspects of the neural function related to behavior.24,25 PUFA n-3 are transferred of the mother to the fetus through the placenta during the intrauterine development.26
The cephalic growth is one of the best ways of neonatal development evaluation. The end of gestation and beginning of extra uterine life are periods of mielinization that lead to a fast increase in the number of cells and in the dendritic area of cerebral tissue. The brain weight is, therefore, associated to its development suggesting that the incorporation of fatty acids to cerebral tissue has influence on this development.27 In this study, the greater mean value of cerebral weight in newborn rats was produced by the maternal diet of flaxseed, having statistic significance when compared to the other diets, casein and modified casein.
In this analysis, the influence of fatty acids proceeding from the flaxseed diet offered to the fetus only by the maternal circulation is being evaluated, since these pups had been submitted to euthanasia immediately after birth to assure the evaluations free from the influence of maternal milk. In the present study, the profile of brain fatty acids in newborn rats showed that the mothers´ pups that consumed the flaxseed diet presented a very high percentage of the fatty acid C 22:5 n-3, as well as in the sum of PUFA, EFA and n-3 with statistical difference when compared to the casein diet. This result is perfectly explained by the high concentration of fatty acids of the n-3 family present in the diet offered to these mothers. The percentile amount of DHA was also very high in the pups´ brain of the flaxseed group (FG), showing statistic significance when compared to the other groups fed with casein diet (CG and MCG); this fact confirms the transference of mothers´ DHA for the fetus during the gestation, as already notified in some studies.12,28,29 It was observed in this study that the supply of flaxseed diet is directly related to the incorporation of fatty acids of the n-3 family in the pups´ brain, and this was well established in the brain composition of acid C 22:6 n-3 (DHA) and in the sum of n-3 (∑-3), since these values were higher in FG and with statistic difference compared to CG and MCG. Similar results were expressed in a study that evaluated pups´ brain of rat born from mothers fed with diets with and without EFA; a higher accumulation of DHA and σn-3 was found on the phospholipids phosphatidylcholine, phosphatidylethanolamine and phosphatidylserine in the pups´ brain proceeding from mothers that had consumed diet with EFA, when compared with the ones that had not consumed it.29
The largest percentage value of relative cerebral weight in the newborns of FG, with statistical significance when compared to the other groups, indicates larger omega-3 fatty acids absorption by this organ, coming from the maternal circulation in this vulnerable period of the Central Nervous System development. 5,30.
Conclusion
The omega-3 fatty acids present in the flaxseed were abundant in the diet made with this seed and the pup´s brain originating from mothers fed with this diet also presented higher percentage of these fatty acids. The use of a diet based on flaxseed during the gestation has influence on the incorporation of omega-3 fatty acids in the brain of newborn rats, contributing to a better cerebral development.
Acknowledgement
The authors are grateful to Dr. Maria das Graças Tavares do Carmo (Josué de Castro Institute of Nutrition, UFRJ, RJ, Brazil) for her technical assistance. Thanks to CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and UFF (Universidade Federal Fluminense) for the institutional support.
References
1. Barker DJP. Mothers, babies and health in later life. London Churchill Livingstone, 1998. [ Links ]
2. Moura AS, Franco Sá CCN, Cruz HG, Costa CL. Malnutrition during lactation as a metabolic imprinting factor inducing the feeding pattern of offspring rats when adults - The role of insulin and leptin. Braz J Med Biol Res 2002; 35:617-622. [ Links ]
3. Simopoulos AP, Bazan NG. Omega-3 fatty acids, the brain and retina. World Rev Nutr Diet 2009; 99: 1-12. [ Links ]
4. Rotenstein L, Herath K, Gould RM, Bellard ME. Characterization of the shark myelin Po protein. Brain Behav Evol 2008; 72: 48-58. [ Links ]
5. Zomignani AP, Zambelli HJL, Antonio MARGM. Desenvolvimento cerebral em recém-nascidos prematuros. Rev Paul Pediatr 2009;27:198-203. [ Links ]
6. Silva DRB, Junior PFM, Soares EA. A importância dos ácidos graxos poliinsaturados de cadeia longa na gestagao e lactação. Rev Bras Saúde Mater Infant 2007; 7: 123-133. [ Links ]
7. Hay WW Jr. Strategies for feeding the preterm infant. Neonatology 2008; 94: 245-254. [ Links ]
8. Levant B, Ozias MK, Davis PF, Winter M, Russel KL, Carlson SE et al. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: Interactions with reproductive status in female rats. Psychoneuroendocrinology 2008; 33: 1279-1292. [ Links ]
9. Gustafson KM, Colombo J, Carlson SE. Docosahexaenoic acid and cognitive function: Is the link mediated by the autonomic nervous system? Prostaglandins Leukot Essent Fatty Acids 2008; 79: 135-140. [ Links ]
10. Yeh YY, Gehman MF, Yeh SM. Maternal dietary fish oil enriches docosahexaenoate levels in brain subcellular fractions of offspring. J Neurosci Res 1993; 35: 218-226. [ Links ]
11. Belkind-Gerson J, Carreón-Rodríguez A, Contreras-Ochoa CO, Estrada-Mondaca S, Parra-Cabrera MS. Fatty Acids and Neurodevelopment. J Pediatr Gastroenterol Nutr 2008; 41:7-9. [ Links ]
12. Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr 2008; 137: 855-859. [ Links ]
13. Rosa DD, Sales RL, Moraes LFS, Lourenço FC, Neves CA, Sabarense CM et al. Flaxseed, olive and fish oil influence plasmatic lipids, lymphocyte migration and morphometry of the intestinal of Wistar rats. Acta Cir Bras 2010; 25: 275-280. [ Links ]
14. Khana G, Penttinem P, Cabanes A, Foxworth A, Chezek A, Mastropole K et al. Maternal flaxseed diet during pregnancy or lactation increases female rat offspring's susceptibility to carcinogen-induced mammary tumorigenesis. Reprod Toxicol 2007; 23: 397-406. [ Links ]
15. Turatti JM. Oleos vegetáis como fonte de alimentos funcionáis. Óleos e Grãos 2000; 56: 20-27. [ Links ]
16. Reeves PG, Nielsen FH, Fahey Jr GC. AIN-93 Purified diet of laboratory rodents: Final report of the American Institute of Nutrition ad hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J Nutr 1993; 123: 1939-1951. [ Links ]
17. Assumpção RP, Santos FD, Barreto GF, Andrade PMM, Tavares do Carmo MG. Effect of variation of trans fatty acid in lac-tating rat's diet on the lipoprotein lipase activity in mammary gland, liver, and adipose tissue. Nutrition 2004; 20: 806-811. [ Links ]
18. Uauy R, Valenzuela A. Marine oils: The health benefits of n-3 fatty acids. Nutrition 2000; 16: 680-684. [ Links ]
19. Morris DH. Flax - A Health and Nutrition Primer (access august 2008). Available from: http://www.flaxcouncil.ca/english/index.php?p=primer&mp=nutrition [ Links ]
20. Visentainer JV, Gomes STM, Hayashi C, Santos-Jr OO, Silva ABM, Justi KC et al. Efeito do tempo de fornecimento de ração suplementada com óleo de linhaça sobre a composição físico-química e de ácidos graxos em cabeças de tilápias do Nilo (Oreochromis niloticus). Cienc Tecnol Aliment 2003; 23: 478-484. [ Links ]
21. Patin RV, Vitólo MR, Valverde MA, Carvalho PO, Pastore GM, Lopez FA. The influence of sardine consumption on the omega-3 fatty acid content of mature human milk. J Pediatr 2006; 82: 63-69. [ Links ]
22. Reinis S, Golman JM. Development of the brain - Biological and functional perspective, Illinois Thomas CC Publishers, 1980. [ Links ]
23. Xiang M, Alfvén G, Blennow M, Trygg M, Zetterstrom R. Long-chain polyunsaturated fatty acids in human milk and brain growth during early infancy. Acta Paediatr 2000; 89: 142-147. [ Links ]
24. Koletzko B, Rodríguez-Palmero A, Demmelmair H, Fildler N, Jensen R, Sauerwald T. Physiological aspects of human milk lipids. Early Hum Dev 2001; 65: 3-18. [ Links ]
25. Brenna JT, Diau GY. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot Essent Fatty Acids 2007; 77: 247-250. [ Links ]
26. Presa-Owens S, López-Sabater MC, Rivero-Urgell M. Fatty acid composition of human milk in Spain. J Pediatr Gastroenterol Nutr 1996; 22: 180-185. [ Links ]
27. Koletzko B, Cetin I, Brenna T. Dietary fat intakes for pregnant and lactating women. Br J Nutr 2007; 98: 873-877. [ Links ]
28. Cocchi M, Pignattic, Carpigiani M, Tarozzi G, Turchetto E. Effect of C 18:3(n-3) dietary supplementation on the fatty acid composition of the rat brain. Acta Vitaminol Enzymol 1984; 6: 151-156. [ Links ]
29. Tinoco SMB, Sichieri R, Moura AS, Santos FS, Carmo MGT. Importância dos ácidos graxos essenciais e os efeitos dos ácidos graxos trans do leite materno para o desenvolvimento fetal e neonatal. Cad Saúde Pública 2007; 23: 525-534. [ Links ]
30. Oken E, Mandy B, Belfort MB. Fish, fish oil, and pregnancy. JAMA 2010; 304: 1717-1718. [ Links ]
31. Lukim WJ, Bazan NG. Docosahexaenoic acid and the aging brain. J Nutr 2008; 138:2510-2514. [ Links ]
![]() Correspondence:
Correspondence:
Katia Calvi Lenzi de Almeida.
E-mail: calvilenzi@gmail.com
Recibido: 21-VII-2010.
1a Revisión: 24-XI-2010
Aceptado: 2-XII-2010.